当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid‐Responsive Transferrin Dissociation and GLUT Mediated Exocytosis for Increased Blood–Brain Barrier Transcytosis and Programmed Glioma Targeting Delivery
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-05-28 , DOI: 10.1002/adfm.201802227 Shaobo Ruan 1 , Lin Qin 1 , Wei Xiao 1 , Chuan Hu 1 , Yang Zhou 1 , Ranran Wang 1 , Xing Sun 1 , Wenqi Yu 1 , Qin He 1 , Huile Gao 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-05-28 , DOI: 10.1002/adfm.201802227 Shaobo Ruan 1 , Lin Qin 1 , Wei Xiao 1 , Chuan Hu 1 , Yang Zhou 1 , Ranran Wang 1 , Xing Sun 1 , Wenqi Yu 1 , Qin He 1 , Huile Gao 1
Affiliation
|
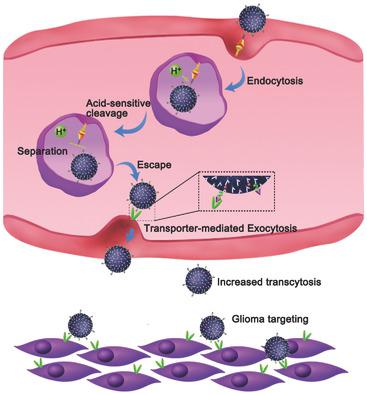
Receptor mediated transcytosis (RMT) is a common mechanism used for nanotherapeutics to traverse the blood–brain barrier (BBB). However, the transcytosis of ligand modified nanoparticles via RMT is likely to be trapped within brain capillary endothelial cells due to the high binding affinity of ligand with receptors, which greatly reduces the amount of nanoparticles across BBB. Here, P‐aminophenyl‐α‐D‐mannopyranoside (MAN) decorated doxorubicin‐loaded dendrigraft poly‐l‐lysine with acid‐cleavable transferrin (Tf) coating outside (DD‐MCT) is proposed. The DD‐MCT is engineered to specifically recognize the Tf receptor (TfR) on the luminal side of BBB endothelium. Then the DD‐MCT undergoes an acid‐responsive cleavage of Tf, leading to the separation of MAN‐decorated DGL‐DOX (DD‐M) from the Tf–TfR complex in endo/lysosomes. The detached DD‐M is more prone to escape from endo/lysosomes and can further be exocytosed into brain parenchyma via the mediation of glucose transporter located on the abluminal endothelial membrane. Moreover, the DD‐M in brain parenchyma can target glioma cells. Significantly, the DD‐MCT enters into brain parenchyma in greater amounts, resulting in enhanced accumulation at glioma site and thus improved antiglioma therapeutic outcome. This strategy pioneers a new path for reducing the trapping of nanotherapeutics within BBB endothelium but increasing their transcytosis into brain parenchyma.
中文翻译:

酸反应性转铁蛋白解离和GLUT介导的胞吐作用增加了血脑屏障的转胞作用和靶向胶质瘤的靶向递送
受体介导的胞吞作用(RMT)是用于纳米疗法穿越血脑屏障(BBB)的常见机制。然而,由于配体与受体的高结合亲和力,经由RMT进行的配体修饰的纳米颗粒的转胞吞作用可能被捕获在脑毛细血管内皮细胞内,这大大减少了整个BBB中纳米颗粒的数量。在这里,提出了用对氨基苯酚-α-D-甘露吡喃糖苷(MAN)装饰的阿霉素负载的树突状移植物聚l-赖氨酸,并在其外部涂有酸可裂解转铁蛋白(Tf)涂层(DD-MCT)。DD‐MCT经过精心设计,可以特异性识别BBB内皮腔侧的Tf受体(TfR)。然后,DD-MCT经历Tf的酸响应裂解,从而导致内膜/溶酶体中MAN装饰的DGL-DOX(DD-M)与Tf-TfR复合物分离。分离的DD-M更容易从内体/溶酶体中逸出,并且可以通过位于腔外内皮膜上的葡萄糖转运蛋白介导而进一步胞外进入脑实质。此外,脑实质中的DD‐M可以靶向神经胶质瘤细胞。值得注意的是,DD-MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善了抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。DD-MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。DD‐MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。
更新日期:2018-05-28
中文翻译:

酸反应性转铁蛋白解离和GLUT介导的胞吐作用增加了血脑屏障的转胞作用和靶向胶质瘤的靶向递送
受体介导的胞吞作用(RMT)是用于纳米疗法穿越血脑屏障(BBB)的常见机制。然而,由于配体与受体的高结合亲和力,经由RMT进行的配体修饰的纳米颗粒的转胞吞作用可能被捕获在脑毛细血管内皮细胞内,这大大减少了整个BBB中纳米颗粒的数量。在这里,提出了用对氨基苯酚-α-D-甘露吡喃糖苷(MAN)装饰的阿霉素负载的树突状移植物聚l-赖氨酸,并在其外部涂有酸可裂解转铁蛋白(Tf)涂层(DD-MCT)。DD‐MCT经过精心设计,可以特异性识别BBB内皮腔侧的Tf受体(TfR)。然后,DD-MCT经历Tf的酸响应裂解,从而导致内膜/溶酶体中MAN装饰的DGL-DOX(DD-M)与Tf-TfR复合物分离。分离的DD-M更容易从内体/溶酶体中逸出,并且可以通过位于腔外内皮膜上的葡萄糖转运蛋白介导而进一步胞外进入脑实质。此外,脑实质中的DD‐M可以靶向神经胶质瘤细胞。值得注意的是,DD-MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善了抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。DD-MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。DD‐MCT大量进入脑实质,导致神经胶质瘤部位积累增加,从而改善抗神经胶质瘤的治疗效果。该策略开创了减少纳米治疗剂在BBB内皮中的捕获但增加其转入脑实质的新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号