当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Chloride Ions on UV/H2O2 and UV/Persulfate Advanced Oxidation Processes
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-06-13 , DOI: 10.1021/acs.est.8b01662 Weiqiu Zhang 1 , Shiqing Zhou 2 , Julong Sun 2 , Xiaoyang Meng 1 , Jinming Luo 1 , Dandan Zhou 3 , John Crittenden 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-06-13 , DOI: 10.1021/acs.est.8b01662 Weiqiu Zhang 1 , Shiqing Zhou 2 , Julong Sun 2 , Xiaoyang Meng 1 , Jinming Luo 1 , Dandan Zhou 3 , John Crittenden 1
Affiliation
|
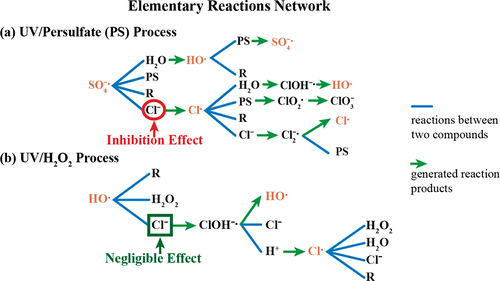
Chloride ion (Cl–) is one of the most common anions in the aqueous environment. A mathematical model was developed to determine and quantify the impact of Cl– on the oxidization rate of organic compounds at the beginning stage of the UV/persulfate (PS) and UV/H2O2 processes. We examined two cases for the UV/PS process: (1) when the target organic compounds react only with sulfate radicals, the ratio of the destruction rate of the target organic compound when Cl– is present to the rate when Cl– is not present (designated as rRCl–/rR) is no larger than 1.942%; and (2) when the target organic compounds can react with sulfate radicals, hydroxyl radicals and chlorine radicals, rRCl–/rR, can be no larger than 60%. Hence, Cl– significantly reduces the organic destruction rate in the UV/PS process. In the UV/H2O2 process, we found that Cl– has a negligible effect on the organic-contaminant oxidation rate. Our simulation results agree with the experimental results very well. Accordingly, our mathematical model is a reliable method for determining whether Cl– will adversely impact organic compounds destruction by the UV/PS and UV/H2O2 processes.
中文翻译:

氯离子对UV / H 2 O 2和UV /过硫酸盐高级氧化过程的影响
氯离子(CL - )是在水性环境中最常见的阴离子之一。开发了一个数学模型来确定和量化在UV /过硫酸盐(PS)和UV / H 2 O 2工艺开始阶段Cl –对有机化合物氧化速率的影响。我们研究了UV / PS过程的两种情况:(1)当目标有机化合物仅与硫酸根自由基反应时,当存在Cl –时目标有机化合物的破坏速率与不存在Cl –时速率的比率。(指定为r R Cl – / r R)不大于1.942%;(2)当目标有机化合物能与硫酸根,羟基和氯基反应时,r R Cl – / r R不得大于60%。因此,Cl –大大降低了UV / PS工艺中的有机破坏率。在UV / H 2 O 2过程中,我们发现Cl –对有机污染物氧化速率的影响可忽略不计。我们的仿真结果与实验结果非常吻合。因此,我们的数学模型是用于确定是否氯一种可靠的方法-将产生不利的有机化合物的破坏通过UV / PS和UV / H影响2 O 2进程。
更新日期:2018-06-14
中文翻译:

氯离子对UV / H 2 O 2和UV /过硫酸盐高级氧化过程的影响
氯离子(CL - )是在水性环境中最常见的阴离子之一。开发了一个数学模型来确定和量化在UV /过硫酸盐(PS)和UV / H 2 O 2工艺开始阶段Cl –对有机化合物氧化速率的影响。我们研究了UV / PS过程的两种情况:(1)当目标有机化合物仅与硫酸根自由基反应时,当存在Cl –时目标有机化合物的破坏速率与不存在Cl –时速率的比率。(指定为r R Cl – / r R)不大于1.942%;(2)当目标有机化合物能与硫酸根,羟基和氯基反应时,r R Cl – / r R不得大于60%。因此,Cl –大大降低了UV / PS工艺中的有机破坏率。在UV / H 2 O 2过程中,我们发现Cl –对有机污染物氧化速率的影响可忽略不计。我们的仿真结果与实验结果非常吻合。因此,我们的数学模型是用于确定是否氯一种可靠的方法-将产生不利的有机化合物的破坏通过UV / PS和UV / H影响2 O 2进程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号