当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability and Placement of Ag/AgCl Quasi-Reference Counter Electrodes in Confined Electrochemical Cells
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.analchem.8b01588 Cameron L. Bentley 1 , David Perry 1 , Patrick R. Unwin 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.analchem.8b01588 Cameron L. Bentley 1 , David Perry 1 , Patrick R. Unwin 1
Affiliation
|
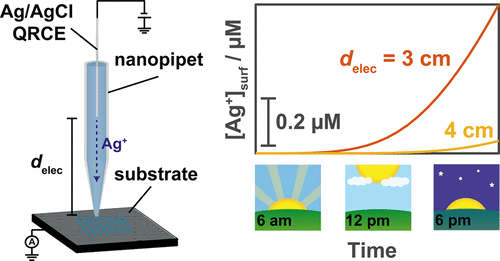
Nanoelectrochemistry is an important and growing branch of electrochemistry that encompasses a number of key research areas, including (electro)catalysis, energy storage, biomedical/environmental sensing, and electrochemical imaging. Nanoscale electrochemical measurements are often performed in confined environments over prolonged experimental time scales with nonisolated quasi-reference counter electrodes (QRCEs) in a simplified two-electrode format. Herein, we consider the stability of commonly used Ag/AgCl QRCEs, comprising an AgCl-coated wire, in a nanopipet configuration, which simulates the confined electrochemical cell arrangement commonly encountered in nanoelectrochemical systems. Ag/AgCl QRCEs possess a very stable reference potential even when used immediately after preparation and, when deployed in Cl– free electrolyte media (e.g., 0.1 M HClO4) in the scanning ion conductance microscopy (SICM) format, drift by only ca. 1 mV h–1 on the several hours time scale. Furthermore, contrary to some previous reports, when employed in a scanning electrochemical cell microscopy (SECCM) format (meniscus contact with a working electrode surface), Ag/AgCl QRCEs do not cause fouling of the surface (i.e., with soluble redox byproducts, such as Ag+) on at least the 6 h time scale, as long as suitable precautions with respect to electrode handling and placement within the nanopipet are observed. These experimental observations are validated through finite element method (FEM) simulations, which consider Ag+ transport within a nanopipet probe in the SECCM and SICM configurations. These results confirm that Ag/AgCl is a stable and robust QRCE in confined electrochemical environments, such as in nanopipets used in SICM, for nanopore measurements, for printing and patterning, and in SECCM, justifying the widespread use of this electrode in the field of nanoelectrochemistry and beyond.
中文翻译:

受限电化学池中Ag / AgCl准参考对电极的稳定性和位置
纳米电化学是电化学领域一个重要且不断发展的分支,它涵盖了许多关键研究领域,包括(电)催化,能量存储,生物医学/环境传感和电化学成像。纳米级电化学测量通常是在狭窄的环境中,以简化的两电极形式使用非隔离的准参考对电极(QRCE)在延长的实验时间范围内进行的。在本文中,我们考虑了纳米吸管配置中包含AgCl涂层导线的常用Ag / AgCl QRCE的稳定性,它模拟了纳米电化学系统中常见的受限电化学电池排列。Ag / AgCl QRCE甚至在制备后立即使用以及部署在Cl –中都具有非常稳定的参考电势。扫描离子电导显微镜(SICM)格式中的游离电解质介质(例如0.1 M HClO 4),仅漂移约。在几个小时的时间刻度上为1 mV h –1。此外,与某些先前的报告相反,当以扫描电化学电池显微镜(SECCM)格式(弯月面与工作电极表面接触)使用时,Ag / AgCl QRCE不会造成表面结垢(即,可溶性氧化还原副产物,例如至少在6小时的时间范围内,如Ag +),只要遵守有关电极处理和在纳米吸管中放置的适当预防措施即可。这些实验观察结果通过有限元方法(FEM)模拟进行了验证,其中考虑了Ag +以SECCM和SICM配置在纳米吸管探针中进行转运。这些结果证实,Ag / AgCl在密闭的电化学环境中(例如,用于SICM的纳米吸管,用于纳米孔的测量,用于印刷和图案化以及在SECCM中)是稳定且坚固的QRCE,证明了该电极在碳纳米管领域的广泛使用是合理的。纳米电化学及其他。
更新日期:2018-05-29
中文翻译:

受限电化学池中Ag / AgCl准参考对电极的稳定性和位置
纳米电化学是电化学领域一个重要且不断发展的分支,它涵盖了许多关键研究领域,包括(电)催化,能量存储,生物医学/环境传感和电化学成像。纳米级电化学测量通常是在狭窄的环境中,以简化的两电极形式使用非隔离的准参考对电极(QRCE)在延长的实验时间范围内进行的。在本文中,我们考虑了纳米吸管配置中包含AgCl涂层导线的常用Ag / AgCl QRCE的稳定性,它模拟了纳米电化学系统中常见的受限电化学电池排列。Ag / AgCl QRCE甚至在制备后立即使用以及部署在Cl –中都具有非常稳定的参考电势。扫描离子电导显微镜(SICM)格式中的游离电解质介质(例如0.1 M HClO 4),仅漂移约。在几个小时的时间刻度上为1 mV h –1。此外,与某些先前的报告相反,当以扫描电化学电池显微镜(SECCM)格式(弯月面与工作电极表面接触)使用时,Ag / AgCl QRCE不会造成表面结垢(即,可溶性氧化还原副产物,例如至少在6小时的时间范围内,如Ag +),只要遵守有关电极处理和在纳米吸管中放置的适当预防措施即可。这些实验观察结果通过有限元方法(FEM)模拟进行了验证,其中考虑了Ag +以SECCM和SICM配置在纳米吸管探针中进行转运。这些结果证实,Ag / AgCl在密闭的电化学环境中(例如,用于SICM的纳米吸管,用于纳米孔的测量,用于印刷和图案化以及在SECCM中)是稳定且坚固的QRCE,证明了该电极在碳纳米管领域的广泛使用是合理的。纳米电化学及其他。































 京公网安备 11010802027423号
京公网安备 11010802027423号