当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
When hydroquinone meets methoxy radical: Hydrogen abstraction reaction from the viewpoint of interacting quantum atoms
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-05-25 , DOI: 10.1002/jcc.25359 Milena Petković 1 , Đura Nakarada 1 , Mihajlo Etinski 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-05-25 , DOI: 10.1002/jcc.25359 Milena Petković 1 , Đura Nakarada 1 , Mihajlo Etinski 1
Affiliation
|
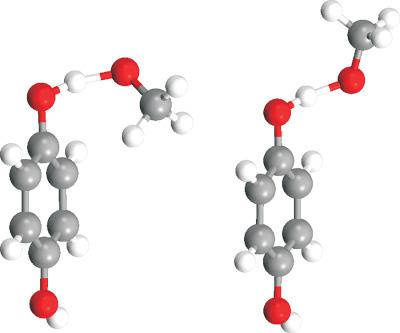
Interacting Quantum Atoms methodology is used for a detailed analysis of hydrogen abstraction reaction from hydroquinone by methoxy radical. Two pathways are analyzed, which differ in the orientation of the reactants at the corresponding transition states. Although the discrepancy between the two barriers amounts to only 2 kJ/mol, which implies that the two pathways are of comparable probability, the extent of intra‐atomic and inter‐atomic energy changes differs considerably. We thus demonstrated that Interacting Quantum Atoms procedure can be applied to unravel distinct energy transfer routes in seemingly similar mechanisms. Identification of energy components with the greatest contribution to the variation of the overall energy (intra‐atomic and inter‐atomic terms that involve hydroquinone's oxygen and the carbon atom covalently bound to it, the transferring hydrogen and methoxy radical's oxygen), is performed using the Relative energy gradient method. Additionally, the Interacting Quantum Fragments approach shed light on the nature of dominant interactions among selected fragments: both Coulomb and exchange‐correlation contributions are of comparable importance when considering interactions of the transferring hydrogen atom with all other atoms, whereas the exchange‐correlation term dominates interaction between methoxy radical's methyl group and hydroquinone's aromatic ring. This study represents one of the first applications of Interacting Quantum Fragments approach on first order saddle points. © 2018 Wiley Periodicals, Inc.
中文翻译:

当对苯二酚遇到甲氧基时:从相互作用的量子原子的角度看夺氢反应
相互作用量子原子方法用于详细分析甲氧基自由基从氢醌中夺氢反应。分析了两种途径,它们在相应过渡态的反应物方向上有所不同。尽管这两个势垒之间的差异仅为 2 kJ/mol,这意味着这两种途径的概率相当,但原子内和原子间能量变化的程度差异很大。因此,我们证明了相互作用的量子原子程序可用于以看似相似的机制解开不同的能量转移路线。识别对整体能量变化贡献最大的能量成分(原子内和原子间术语,涉及对苯二酚的氧和与其共价结合的碳原子,转移氢和甲氧基的氧),使用相对能量梯度法进行。此外,相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:在考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项占主导地位甲氧基的甲基与氢醌的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:当考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项支配了之间的相互作用甲氧基的甲基和对苯二酚的芳环。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:当考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项支配了之间的相互作用甲氧基的甲基和对苯二酚的芳环。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 而交换相关项支配着甲氧基的甲基和对苯二酚的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 而交换相关项支配着甲氧基的甲基和对苯二酚的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc.
更新日期:2018-05-25
中文翻译:

当对苯二酚遇到甲氧基时:从相互作用的量子原子的角度看夺氢反应
相互作用量子原子方法用于详细分析甲氧基自由基从氢醌中夺氢反应。分析了两种途径,它们在相应过渡态的反应物方向上有所不同。尽管这两个势垒之间的差异仅为 2 kJ/mol,这意味着这两种途径的概率相当,但原子内和原子间能量变化的程度差异很大。因此,我们证明了相互作用的量子原子程序可用于以看似相似的机制解开不同的能量转移路线。识别对整体能量变化贡献最大的能量成分(原子内和原子间术语,涉及对苯二酚的氧和与其共价结合的碳原子,转移氢和甲氧基的氧),使用相对能量梯度法进行。此外,相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:在考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项占主导地位甲氧基的甲基与氢醌的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:当考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项支配了之间的相互作用甲氧基的甲基和对苯二酚的芳环。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 相互作用的量子碎片方法阐明了选定碎片之间主要相互作用的性质:当考虑转移氢原子与所有其他原子的相互作用时,库仑和交换相关贡献都具有相当的重要性,而交换相关项支配了之间的相互作用甲氧基的甲基和对苯二酚的芳环。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 而交换相关项支配着甲氧基的甲基和对苯二酚的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc. 而交换相关项支配着甲氧基的甲基和对苯二酚的芳环之间的相互作用。这项研究代表了相互作用量子碎片方法在一阶鞍点上的首批应用之一。© 2018 Wiley Periodicals, Inc.















































 京公网安备 11010802027423号
京公网安备 11010802027423号