当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-03-31 00:00:00 , DOI: 10.1021/cs502128q Dan Ren 1 , Yilin Deng 1 , Albertus Denny Handoko 1 , Chung Shou Chen 1 , Souradip Malkhandi 1 , Boon Siang Yeo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-03-31 00:00:00 , DOI: 10.1021/cs502128q Dan Ren 1 , Yilin Deng 1 , Albertus Denny Handoko 1 , Chung Shou Chen 1 , Souradip Malkhandi 1 , Boon Siang Yeo 1
Affiliation
|
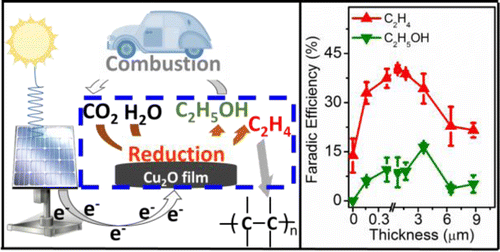
The selective electroreduction of carbon dioxide to C2 compounds (ethylene and ethanol) on copper(I) oxide films has been investigated at various electrochemical potentials. Aqueous 0.1 M KHCO3 was used as electrolyte. A remarkable finding is that the faradic yields of ethylene and ethanol can be systematically tuned by changing the thickness of the deposited overlayers. Films 1.7–3.6 μm thick exhibited the best selectivity for these C2 compounds at −0.99 V vs RHE, with faradic efficiencies (FE) of 34–39% for ethylene and 9–16% for ethanol. Less than 1% methane was formed. A high C2H4/CH4 products’ ratio of up to ∼100 could be achieved. Scanning electron microscopy, X-ray diffraction, and in situ Raman spectroscopy revealed that the Cu2O films reduced rapidly and remained as metallic Cu0 particles during the CO2 reduction. The selectivity trends exhibited by the catalysts during CO2 reduction in phosphate buffer, and KHCO3 electrolytes suggest that an increase in local pH at the surface of the electrode is not the only factor in enhancing the formation of C2 products. An optimized surface population of edges and steps on the catalyst is also necessary to facilitate the dissociation of CO2 and the dimerization of the pertinent CHxO intermediates to ethylene and ethanol.
中文翻译:

在氧化铜(I)催化剂上将二氧化碳选择性电化学还原为乙烯和乙醇
在各种电化学势下,已经研究了在氧化铜(I)薄膜上将二氧化碳选择性电还原为C 2化合物(乙烯和乙醇)的方法。使用0.1M KHCO 3水溶液作为电解质。一个显着的发现是,可以通过改变沉积的叠层的厚度来系统地调节乙烯和乙醇的法拉第产率。厚度为1.7-3.6μm的薄膜在-0.99 V相对于RHE的情况下,对这些C 2化合物表现出最佳的选择性,乙烯的法拉第效率(FE)为34-39%,乙醇的法拉第效率(FE)为9-16%。形成的甲烷少于1%。高C 2 H 4 / CH 4产品的比率可以达到约100。扫描电子显微镜,X射线衍射和原位拉曼光谱显示,Cu 2 O膜迅速还原并在CO 2还原过程中保留为金属Cu 0颗粒。在磷酸盐缓冲液中的CO 2还原过程中,催化剂表现出的选择性趋势,而KHCO 3电解质表明,电极表面局部pH的升高并不是增强C 2产物形成的唯一因素。为了促进CO 2的解离和相关CH的二聚化,还需要优化催化剂表面的边缘和台阶的表面填充量。× O为乙烯和乙醇的中间体。
更新日期:2015-03-31
中文翻译:

在氧化铜(I)催化剂上将二氧化碳选择性电化学还原为乙烯和乙醇
在各种电化学势下,已经研究了在氧化铜(I)薄膜上将二氧化碳选择性电还原为C 2化合物(乙烯和乙醇)的方法。使用0.1M KHCO 3水溶液作为电解质。一个显着的发现是,可以通过改变沉积的叠层的厚度来系统地调节乙烯和乙醇的法拉第产率。厚度为1.7-3.6μm的薄膜在-0.99 V相对于RHE的情况下,对这些C 2化合物表现出最佳的选择性,乙烯的法拉第效率(FE)为34-39%,乙醇的法拉第效率(FE)为9-16%。形成的甲烷少于1%。高C 2 H 4 / CH 4产品的比率可以达到约100。扫描电子显微镜,X射线衍射和原位拉曼光谱显示,Cu 2 O膜迅速还原并在CO 2还原过程中保留为金属Cu 0颗粒。在磷酸盐缓冲液中的CO 2还原过程中,催化剂表现出的选择性趋势,而KHCO 3电解质表明,电极表面局部pH的升高并不是增强C 2产物形成的唯一因素。为了促进CO 2的解离和相关CH的二聚化,还需要优化催化剂表面的边缘和台阶的表面填充量。× O为乙烯和乙醇的中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号