当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of (R)-1-(3-(4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)-2-(dimethylamino)ethanone (CHMFL-FLT3-122) as a Potent and Orally Available FLT3 Kinase Inhibitor for FLT3-ITD Positive Acute Myeloid Leukemia
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-12-14 00:00:00 , DOI: 10.1021/acs.jmedchem.5b01611 Xixiang Li 1, 2 , Aoli Wang 1, 3 , Kailin Yu 1, 3 , Ziping Qi 1, 2 , Cheng Chen 1, 2 , Wenchao Wang 1, 2 , Chen Hu 1, 2 , Hong Wu 1, 3 , Jiaxin Wu 1, 3 , Zheng Zhao 1, 2 , Juan Liu 1, 2 , Fengming Zou 1, 2 , Li Wang 1, 2 , Beilei Wang 1, 2 , Wei Wang 1, 2 , Shanchun Zhang 2, 4 , Jing Liu 1, 2 , Qingsong Liu 1, 2, 3, 5
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2015-12-14 00:00:00 , DOI: 10.1021/acs.jmedchem.5b01611 Xixiang Li 1, 2 , Aoli Wang 1, 3 , Kailin Yu 1, 3 , Ziping Qi 1, 2 , Cheng Chen 1, 2 , Wenchao Wang 1, 2 , Chen Hu 1, 2 , Hong Wu 1, 3 , Jiaxin Wu 1, 3 , Zheng Zhao 1, 2 , Juan Liu 1, 2 , Fengming Zou 1, 2 , Li Wang 1, 2 , Beilei Wang 1, 2 , Wei Wang 1, 2 , Shanchun Zhang 2, 4 , Jing Liu 1, 2 , Qingsong Liu 1, 2, 3, 5
Affiliation
|
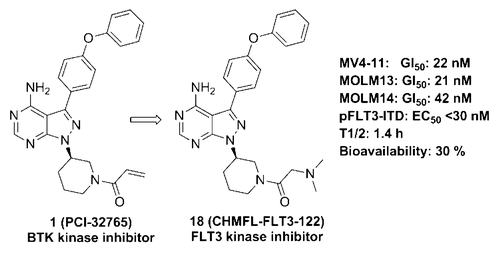
FLT3-ITD mutant has been observed in about 30% of AML patients and extensively studied as a drug discovery target. On the basis of the structure of PCI-32765 (ibrutinib), a BTK kinase inhibitor that was recently reported to bear FLT3 kinase activity through a structure-guided drug design approach, we have discovered compound 18 (CHMFL-FLT3-122), which displayed an IC50 of 40 nM against FLT3 kinase and achieved selectivity over BTK kinase (over 10-fold). It significantly inhibited the proliferation of FLT3-ITD positive AML cancer cell lines MV4-11 (GI50 = 22 nM), MOLM13/14 (GI50 = 21 nM/42 nM). More importantly, 18 demonstrated 170-fold selectivity between FLT3 kinase and c-KIT kinase (GI50 = 11 nM versus 1900 nM) in the TEL-fusion isogenic BaF3 cells indicating a potential to avoid the FLT3/c-KIT dual inhibition induced myelosuppression toxicity. In the cellular context it strongly affected FLT3-ITD mediated signaling pathways and induced apoptosis by arresting the cell cycle into the G0/G1 phase. In the in vivo studies 18 demonstrated a good bioavailability (30%) and significantly suppressed the tumor growth in MV4-11 cell inoculated xenograft model (50 mg/kg) without exhibiting obvious toxicity. Compound 18 might be a potential drug candidate for FLT3-ITD positive AML.
中文翻译:

(R)-1-(3-(4-氨基-3-(4-苯氧基苯基)-1 H-吡唑并[3,4- d ]嘧啶-1-基)哌啶-1-基)-2-的发现(二甲氨基)乙酮(CHMFL-FLT3-122)作为有效且可口服的FLT3-ITD阳性急性髓细胞白血病的FLT3激酶抑制剂
已经在大约30%的AML患者中观察到了FLT3-ITD突变体,并将其作为药物发现靶标进行了广泛的研究。根据最近报道通过结构指导药物设计方法具有FLT3激酶活性的BTK激酶抑制剂PCI-32765(ibrutinib)的结构,我们发现了化合物18(CHMFL-FLT3-122),对FLT3激酶的IC 50为40 nM,相对于BTK激酶具有选择性(超过10倍)。它显着抑制了FLT3-ITD阳性AML癌细胞系MV4-11(GI 50 = 22 nM),MOLM13 / 14(GI 50 = 21 nM / 42 nM)的增殖。更重要的是,有18个在FLT3激酶和c-KIT激酶之间显示出170倍的选择性(GI 50在tel-fusion等基因BaF3细胞中,其相对于1900 nM分别为11 nM和1900 nM),表明有可能避免FLT3 / c-KIT双重抑制诱导的骨髓抑制毒性。在细胞环境中,它通过阻止细胞周期进入G0 / G1期,强烈影响FLT3-ITD介导的信号通路并诱导凋亡。在体内研究中18显示出良好的生物利用度(30%),并在MV4-11细胞接种的异种移植模型(50 mg / kg)中显着抑制了肿瘤的生长,而没有表现出明显的毒性。化合物18可能是FLT3-ITD阳性AML的潜在候选药物。
更新日期:2015-12-14
中文翻译:

(R)-1-(3-(4-氨基-3-(4-苯氧基苯基)-1 H-吡唑并[3,4- d ]嘧啶-1-基)哌啶-1-基)-2-的发现(二甲氨基)乙酮(CHMFL-FLT3-122)作为有效且可口服的FLT3-ITD阳性急性髓细胞白血病的FLT3激酶抑制剂
已经在大约30%的AML患者中观察到了FLT3-ITD突变体,并将其作为药物发现靶标进行了广泛的研究。根据最近报道通过结构指导药物设计方法具有FLT3激酶活性的BTK激酶抑制剂PCI-32765(ibrutinib)的结构,我们发现了化合物18(CHMFL-FLT3-122),对FLT3激酶的IC 50为40 nM,相对于BTK激酶具有选择性(超过10倍)。它显着抑制了FLT3-ITD阳性AML癌细胞系MV4-11(GI 50 = 22 nM),MOLM13 / 14(GI 50 = 21 nM / 42 nM)的增殖。更重要的是,有18个在FLT3激酶和c-KIT激酶之间显示出170倍的选择性(GI 50在tel-fusion等基因BaF3细胞中,其相对于1900 nM分别为11 nM和1900 nM),表明有可能避免FLT3 / c-KIT双重抑制诱导的骨髓抑制毒性。在细胞环境中,它通过阻止细胞周期进入G0 / G1期,强烈影响FLT3-ITD介导的信号通路并诱导凋亡。在体内研究中18显示出良好的生物利用度(30%),并在MV4-11细胞接种的异种移植模型(50 mg / kg)中显着抑制了肿瘤的生长,而没有表现出明显的毒性。化合物18可能是FLT3-ITD阳性AML的潜在候选药物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号