当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-Temperature CO2 Methanation over CeO2-Supported Ru Single Atoms, Nanoclusters, and Nanoparticles Competitively Tuned by Strong Metal–Support Interactions and H-Spillover Effect
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1021/acscatal.7b04469 Yu Guo , Sheng Mei , Kun Yuan , De-Jiu Wang , Hai-Chao Liu , Chun-Hua Yan , Ya-Wen Zhang
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1021/acscatal.7b04469 Yu Guo , Sheng Mei , Kun Yuan , De-Jiu Wang , Hai-Chao Liu , Chun-Hua Yan , Ya-Wen Zhang
|
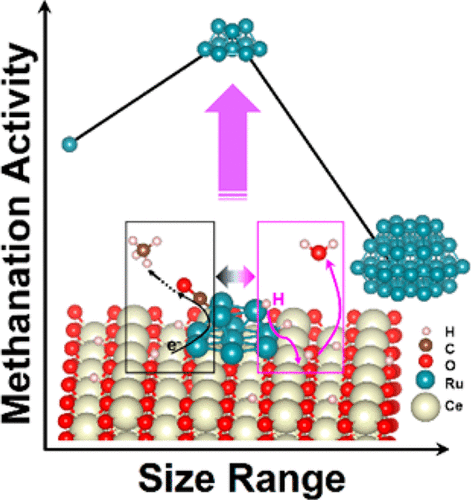
CO2 hydrogenation for the acquisition of value-added chemicals is an economical means to deal with the CO2-relevant environmental problems, among which CO2 reduction to CH4 is an excellent model reaction for investigating the initial steps of CO2 hydrogenation. For the supported catalysts commonly used in such reactions, the tailoring of the interfacial effect between metal centers and supporting materials so as to obtain superior low-temperature CO2 methanation performance is a significant but challenging subject. In this work, we altered the size regimes of the Ru deposits in Ru/CeO2 assemblies and uncovered the competitive relationship between the strong metal–support interactions (SMSI) and the H-spillover effect in determining the methanation activities by some ex situ and in situ spectroscopic techniques coupled with density functional theory (DFT) calculations. For CeO2 nanowire supported single Ru atoms, Ru nanoclusters (ca. 1.2 nm in size), and large Ru nanoparticles (ca. 4.0 nm in size), the nanoclusters show the most outstanding low-temperature CO2 methanation activity and 98–100% selectivity, with a turnover frequency (TOF) of 7.41 × 10–3 s–1 at 190 °C. The negative CO2 reaction order decreases their absolute values from single atoms to nanoclusters and turns positive in nanoparticles, while the positive H2 reaction order follows the reverse tendency. In situ DRIFTS measurements demonstrate that the dominant reaction pathway is the CO route, in which metal carbonyls are the critical intermediates and the active sites are those Ce3+–OH sites and Ru sites near the metal–support interfaces in charge of CO2 dissociation and carbonyl hydrogenation, respectively. Meanwhile, the strongest SMSI and H-spillover effect are respectively encountered in supported single Ru atoms and large Ru nanoparticles, with the activation of metal carbonyls and the dehydration of the support surfaces suppressed correspondingly. The two factors reach a balance in CeO2-supported Ru nanoclusters, and the methanation activity is therefore maximized. A mechanistic understanding of the interfacial effect in tuning the CO2 methanation activities would shed light on the ingenious design of the CO2 hydrogenation catalysts to utilize the SMSI and H-spillover effect to an appropriate degree and avoid their possible suppressions that would take place in extreme cases.
中文翻译:

CeO 2支撑的Ru单原子,纳米团簇和纳米粒子的低温CO 2甲烷化,可通过强大的金属-支撑相互作用和H溢出效应进行竞争性调节
用于获得增值化学品的CO 2加氢是解决与CO 2相关的环境问题的经济方法,其中将CO 2还原为CH 4是研究CO 2加氢初始步骤的出色模型反应。对于通常用于此类反应的负载型催化剂,调整金属中心与载体材料之间的界面效应,以获得优异的低温CO 2甲烷化性能是一个重要但具有挑战性的课题。在这项工作中,我们改变了Ru / CeO 2中Ru沉积物的尺寸范围组装并揭示了通过一些非原位和原位光谱技术结合密度泛函理论(DFT)计算确定甲烷化活性时,强金属-支撑相互作用(SMSI)和H溢出效应之间的竞争关系。对于CeO 2纳米线支持的单个Ru原子,Ru纳米簇(尺寸约1.2 nm)和大Ru纳米颗粒(尺寸约4.0 nm),纳米簇显示出最出色的低温CO 2甲烷化活性和98–100选择性为%,在190°C下的转换频率(TOF)为7.41×10 –3 s –1。负CO 2H 2反应阶数从单原子到纳米团簇的绝对值减小,并在纳米粒子中变为正,而H 2的正反应级数遵循相反的趋势。原位DRIFTS测量表明,主要的反应途径是CO途径,其中羰基金属是关键中间体,而活性位点是负责CO 2的金属-载体界面附近的那些Ce 3+ -OH位点和Ru位点离解和羰基氢化。同时,在负载的单个Ru原子和较大的Ru纳米粒子中分别遇到最强的SMSI和H溢出效应,相应地抑制了羰基金属的活化和载体表面的脱水。这两个因素在CeO 2负载的Ru纳米团簇中达到平衡,因此甲烷化活性达到最大。对调整CO 2甲烷化活性的界面效应的机械理解将为CO 2加氢催化剂的巧妙设计提供启示,以在适当的程度上利用SMSI和H溢出效应,并避免它们可能在反应中发生的抑制作用。极端的情况。
更新日期:2018-05-24
中文翻译:

CeO 2支撑的Ru单原子,纳米团簇和纳米粒子的低温CO 2甲烷化,可通过强大的金属-支撑相互作用和H溢出效应进行竞争性调节
用于获得增值化学品的CO 2加氢是解决与CO 2相关的环境问题的经济方法,其中将CO 2还原为CH 4是研究CO 2加氢初始步骤的出色模型反应。对于通常用于此类反应的负载型催化剂,调整金属中心与载体材料之间的界面效应,以获得优异的低温CO 2甲烷化性能是一个重要但具有挑战性的课题。在这项工作中,我们改变了Ru / CeO 2中Ru沉积物的尺寸范围组装并揭示了通过一些非原位和原位光谱技术结合密度泛函理论(DFT)计算确定甲烷化活性时,强金属-支撑相互作用(SMSI)和H溢出效应之间的竞争关系。对于CeO 2纳米线支持的单个Ru原子,Ru纳米簇(尺寸约1.2 nm)和大Ru纳米颗粒(尺寸约4.0 nm),纳米簇显示出最出色的低温CO 2甲烷化活性和98–100选择性为%,在190°C下的转换频率(TOF)为7.41×10 –3 s –1。负CO 2H 2反应阶数从单原子到纳米团簇的绝对值减小,并在纳米粒子中变为正,而H 2的正反应级数遵循相反的趋势。原位DRIFTS测量表明,主要的反应途径是CO途径,其中羰基金属是关键中间体,而活性位点是负责CO 2的金属-载体界面附近的那些Ce 3+ -OH位点和Ru位点离解和羰基氢化。同时,在负载的单个Ru原子和较大的Ru纳米粒子中分别遇到最强的SMSI和H溢出效应,相应地抑制了羰基金属的活化和载体表面的脱水。这两个因素在CeO 2负载的Ru纳米团簇中达到平衡,因此甲烷化活性达到最大。对调整CO 2甲烷化活性的界面效应的机械理解将为CO 2加氢催化剂的巧妙设计提供启示,以在适当的程度上利用SMSI和H溢出效应,并避免它们可能在反应中发生的抑制作用。极端的情况。































 京公网安备 11010802027423号
京公网安备 11010802027423号