当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.est.8b00959 Eun-Tae Yun 1 , Jeong Hoon Lee 1 , Jaesung Kim 1 , Hee-Deung Park 1 , Jaesang Lee 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.est.8b00959 Eun-Tae Yun 1 , Jeong Hoon Lee 1 , Jaesung Kim 1 , Hee-Deung Park 1 , Jaesang Lee 1
Affiliation
|
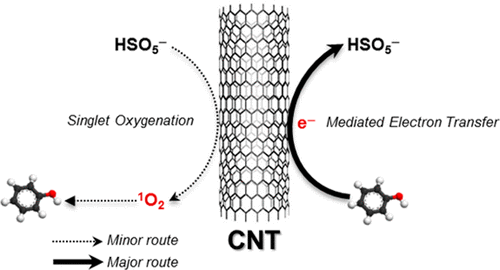
Select persulfate activation processes were demonstrated to initiate oxidation not reliant on sulfate radicals, although the underlying mechanism has yet to be identified. This study explored singlet oxygenation and mediated electron transfer as plausible nonradical mechanisms for organic degradation by carbon nanotube (CNT)-activated peroxymonosulfate (PMS). The degradation of furfuryl alcohol (FFA) as a singlet oxygen (1O2) indicator and the kinetic retardation of FFA oxidation in the presence of l-histidine and azide as 1O2 quenchers apparently supported a role of 1O2 in the CNT/PMS system. However, the 1O2 scavenging effect was ascribed to a rapid PMS depletion by l-histidine and azide. A comparison of CNT/PMS and photoexcited Rose Bengal (RB) excluded the possibility of singlet oxygenation during heterogeneous persulfate activation. In contrast to the case of excited RB, solvent exchange (H2O to D2O) did not enhance FFA degradation by CNT/PMS and the pH- and substrate-dependent reactivity of CNT/PMS did not reflect the selective nature of 1O2. Alternatively, concomitant PMS reduction and trichlorophenol oxidation were achieved when PMS and trichlorophenol were physically separated in two chambers using a conductive vertically aligned CNT membrane. This result suggested that CNT-mediated electron transfer from organics to persulfate was primarily responsible for the nonradical degradative route.
中文翻译:

识别过氧单硫酸盐活化过程中的非自由基机理:单重态氧与介导的电子转移
尽管尚未确定潜在的机制,但已证明选择的过硫酸盐活化过程可引发不依赖硫酸根的氧化反应。这项研究探讨了单线态氧合和介导的电子转移,作为通过碳纳米管(CNT)活化的过氧一硫酸盐(PMS)进行有机降解的合理的非自由基机理。糠醇(FFA)作为单线态氧(1 O 2)指示剂的降解以及在以1组氨酸和叠氮化物作为1 O 2猝灭剂的情况下FFA氧化的动力学阻滞显然支持了CNT中1 O 2的作用。/ PMS系统。但是,1 O 2清除作用归因于1-组氨酸和叠氮化物的快速PMS消耗。CNT / PMS与光激发的玫瑰红(RB)的比较排除了过硫酸盐活化过程中单线态氧合的可能性。与激发的RB相比,溶剂交换(从H 2 O到D 2 O)不会增强CNT / PMS对FFA的降解,并且CNT / PMS的pH和底物依赖性反应不能反映1 Ø 2。或者,当使用导电垂直排列的CNT膜在两个腔室中物理分离PMS和三氯苯酚时,可以实现伴随的PMS还原和三氯苯酚氧化。该结果表明,CNT介导的电子从有机物到过硫酸盐的转移是造成非自由基降解途径的主要原因。
更新日期:2018-06-01
中文翻译:

识别过氧单硫酸盐活化过程中的非自由基机理:单重态氧与介导的电子转移
尽管尚未确定潜在的机制,但已证明选择的过硫酸盐活化过程可引发不依赖硫酸根的氧化反应。这项研究探讨了单线态氧合和介导的电子转移,作为通过碳纳米管(CNT)活化的过氧一硫酸盐(PMS)进行有机降解的合理的非自由基机理。糠醇(FFA)作为单线态氧(1 O 2)指示剂的降解以及在以1组氨酸和叠氮化物作为1 O 2猝灭剂的情况下FFA氧化的动力学阻滞显然支持了CNT中1 O 2的作用。/ PMS系统。但是,1 O 2清除作用归因于1-组氨酸和叠氮化物的快速PMS消耗。CNT / PMS与光激发的玫瑰红(RB)的比较排除了过硫酸盐活化过程中单线态氧合的可能性。与激发的RB相比,溶剂交换(从H 2 O到D 2 O)不会增强CNT / PMS对FFA的降解,并且CNT / PMS的pH和底物依赖性反应不能反映1 Ø 2。或者,当使用导电垂直排列的CNT膜在两个腔室中物理分离PMS和三氯苯酚时,可以实现伴随的PMS还原和三氯苯酚氧化。该结果表明,CNT介导的电子从有机物到过硫酸盐的转移是造成非自由基降解途径的主要原因。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号