当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophobic Collapse of Ubiquitin Generates Rapid Protein–Water Motions
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00235 Hanna Wirtz 1 , Sarah Schäfer 1 , Claudius Hoberg 1 , Korey M. Reid 2 , David M. Leitner 2 , Martina Havenith 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00235 Hanna Wirtz 1 , Sarah Schäfer 1 , Claudius Hoberg 1 , Korey M. Reid 2 , David M. Leitner 2 , Martina Havenith 1
Affiliation
|
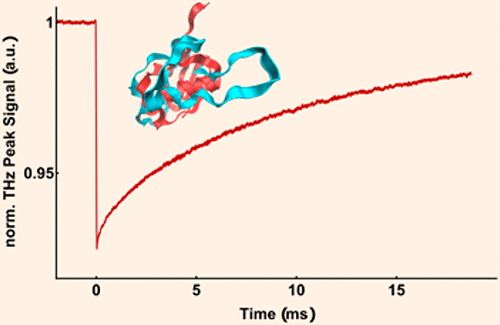
We report time-resolved measurements of the coupled protein–water modes of solvated ubiquitin during protein folding. Kinetic terahertz absorption (KITA) spectroscopy serves as a label-free technique for monitoring large scale conformational changes and folding of proteins subsequent to a sudden T-jump. We report here KITA measurements at an unprecedented time resolution of 500 ns, a resolution 2 orders of magnitude better than those of any previous KITA measurements, which reveal the coupled ubiquitin–solvent dynamics even in the initial phase of hydrophobic collapse. Complementary equilibrium experiments and molecular simulations of ubiquitin solutions are performed to clarify non-equilibrium contributions and reveal the molecular picture upon a change in structure, respectively. On the basis of our results, we propose that in the case of ubiquitin a rapid (<500 ns) initial phase of the hydrophobic collapse from the elongated protein to a molten globule structure precedes secondary structure formation. We find that these very first steps, including large-amplitude changes within the unfolded manifold, are accompanied by a rapid (<500 ns) pronounced change of the coupled protein–solvent response. The KITA response upon secondary structure formation exhibits an opposite sign, which indicates a distinct effect on the solvent-exposed surface.
中文翻译:

泛素的疏水塌陷产生快速的蛋白质-水运动
我们报告了蛋白折叠过程中溶剂化遍在蛋白的耦合蛋白-水模式的时间分辨测量。动力学太赫兹吸收(KITA)光谱是一种无标记技术,可用于监测大规模构象变化和突然T后蛋白质的折叠-跳。我们在这里报告了以500 ns的前所未有的时间分辨率进行的KITA测量,该分辨率比以前的任何KITA测量都高2个数量级,这揭示了即使在疏水塌陷的初始阶段,泛素-溶剂的耦合动力学也是如此。进行泛素溶液的互补平衡实验和分子模拟,以阐明非平衡的贡献并揭示结构变化时的分子图。根据我们的结果,我们建议在泛素的情况下,快速的(<500 ns)初始阶段的疏水塌陷从伸长的蛋白质到熔融的球状结构先于二级结构形成。我们发现,这些第一步,包括展开的歧管内的大幅度变化,都伴随着快速的(< 500 ns)显着改变了蛋白质-溶剂反应的耦合。二级结构形成时的KITA响应显示相反的符号,表明对暴露于溶剂的表面有明显的影响。
更新日期:2018-05-23
中文翻译:

泛素的疏水塌陷产生快速的蛋白质-水运动
我们报告了蛋白折叠过程中溶剂化遍在蛋白的耦合蛋白-水模式的时间分辨测量。动力学太赫兹吸收(KITA)光谱是一种无标记技术,可用于监测大规模构象变化和突然T后蛋白质的折叠-跳。我们在这里报告了以500 ns的前所未有的时间分辨率进行的KITA测量,该分辨率比以前的任何KITA测量都高2个数量级,这揭示了即使在疏水塌陷的初始阶段,泛素-溶剂的耦合动力学也是如此。进行泛素溶液的互补平衡实验和分子模拟,以阐明非平衡的贡献并揭示结构变化时的分子图。根据我们的结果,我们建议在泛素的情况下,快速的(<500 ns)初始阶段的疏水塌陷从伸长的蛋白质到熔融的球状结构先于二级结构形成。我们发现,这些第一步,包括展开的歧管内的大幅度变化,都伴随着快速的(< 500 ns)显着改变了蛋白质-溶剂反应的耦合。二级结构形成时的KITA响应显示相反的符号,表明对暴露于溶剂的表面有明显的影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号