当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfonium Ion Condensation: The Burden Borne by SAM Synthetase
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.biochem.8b00477
Charles A. Lewis 1 , Richard Wolfenden 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.biochem.8b00477
Charles A. Lewis 1 , Richard Wolfenden 1
Affiliation
|
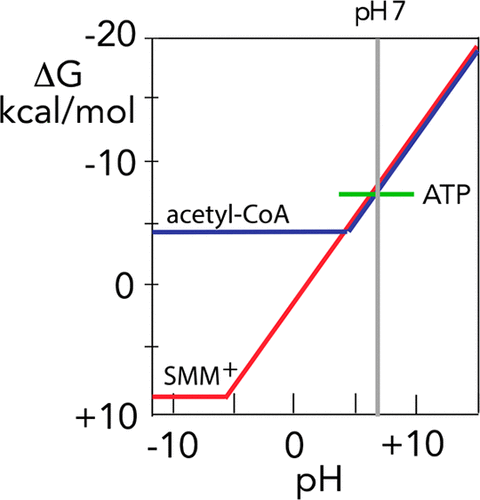
S-Adenosylmethionine (SAM+) serves as the principal methylating agent in biological systems, but the thermodynamic basis of its reactivity does not seem to have been clearly established. Here, we show that methionine, methanol, and H+ combine to form S-methylmethionine (SMM+) with a temperature-independent equilibrium constant of 9.9 M–2. The corresponding group transfer potential of SMM+ (its free energy of hydrolysis at pH 7) is −8.2 kcal/mol. The “energy-rich” nature of sulfonium ions is related to the extreme acidity (pKa −5.4) of the S-protonated thioether produced by sulfonium hydrolysis, and the large negative free energy of deprotonation of that species in neutral solution (−16.7 kcal/mol). At pH 7, SAM synthetase requires the free energy released by cleavage of two bonds of ATP to reverse that process.
中文翻译:

ulf离子冷凝:SAM合成酶带来的负担
S-腺苷甲硫氨酸(SAM +)是生物系统中的主要甲基化剂,但似乎尚未明确其反应性的热力学基础。在这里,我们显示蛋氨酸,甲醇和H +结合形成S-甲基蛋氨酸(SMM +),其温度独立的平衡常数为9.9 M –2。SMM +的相应基团转移电势(其在pH 7时的水解自由能)为-8.2 kcal / mol。sulf离子的“富能”性质与极端酸性相关(p K a-5.4)通过sulf水解产生的S-质子化的硫醚,以及该物种在中性溶液中(-16.7 kcal / mol)的大量负质子化的负自由能。在pH值为7时,SAM合成酶需要通过裂解ATP的两个键释放的自由能来逆转该过程。
更新日期:2018-05-22
中文翻译:

ulf离子冷凝:SAM合成酶带来的负担
S-腺苷甲硫氨酸(SAM +)是生物系统中的主要甲基化剂,但似乎尚未明确其反应性的热力学基础。在这里,我们显示蛋氨酸,甲醇和H +结合形成S-甲基蛋氨酸(SMM +),其温度独立的平衡常数为9.9 M –2。SMM +的相应基团转移电势(其在pH 7时的水解自由能)为-8.2 kcal / mol。sulf离子的“富能”性质与极端酸性相关(p K a-5.4)通过sulf水解产生的S-质子化的硫醚,以及该物种在中性溶液中(-16.7 kcal / mol)的大量负质子化的负自由能。在pH值为7时,SAM合成酶需要通过裂解ATP的两个键释放的自由能来逆转该过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号