Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Infrared Photoluminescence and Reversible Trans-to-Cis Photoisomerization of Mononuclear and Binuclear Ytterbium(III) Complexes Functionalized by Azobenzene Groups
ACS Omega ( IF 3.7 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acsomega.8b00386 Yun-Guang Wang 1 , Yu-Qian Li 1 , Hui-Hui Tang 1 , Li-Rong Lin 1 , Li-Hua Ma 2
ACS Omega ( IF 3.7 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acsomega.8b00386 Yun-Guang Wang 1 , Yu-Qian Li 1 , Hui-Hui Tang 1 , Li-Rong Lin 1 , Li-Hua Ma 2
Affiliation
|
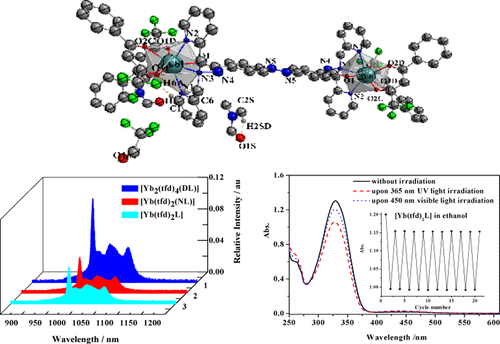
Two mononuclear and one binuclear ytterbium complexes with dual near-infrared (NIR) photoluminescence and reversible trans-to-cis photoisomerization functions were synthesized and characterized. The central ytterbium(III) ion coordinates with two β-diketonate (4,4,4-trifluoro-1-phenylbutane-1,3-dionate (tfd)) ligands and one deprotonated azobenzene-containing tetradentate ligand [(E)-4-(phenyldiazenyl)-N,N-bis(pyridin-2-ylmethyl) benzohydrazide (HL), (E)-4-((4-(dimethylamino)phenyl)diazenyl)-N,N-bis(pyridin-2-ylmethyl)benzohydrazide (HNL), or (E)-4,4′-N′,N′-bis(pyridin-2-ylmethyl)benzohydrazide azobenzene (H2DL)] to form a neutral ternary complex ([Yb(tfd)2L], [Yb(tfd)2(NL)], or [Yb2(tfd)4(DL)], respectively), where the ytterbium(III) ion is eight-coordinated to N3O5 donor sets. X-ray crystallographic analysis shows that all three complexes form a trigonal dodecahedron geometry with similar −N═N– distances that are slightly longer than those of the pure azobenzene-containing ligands. The NIR luminescence properties of the Yb(III) complexes were determined at a wavelength of about 980 nm with quantum yields in the range of 0.4–0.6% in ethanol and acetonitrile solutions at room temperature, and trans-to-cis photoisomerization was determined with the quantum yields (Φt→c = 10–2) at the same level as their pure ligands. The trans-to-cis photoisomerization rates of the complexes (10–4 s–1) are slightly higher than those of the pure ligands and similar to azobenzene (10–5 to 10–4 s–1). From time-dependent density functional theory calculations of the energy levels of the first excited triplet states of the ligands, the energies of the lowest excited triplet states of all of the ligands are higher than the resonance level of Yb3+ (2F5/2, 1.2722 eV). We suggest that these azo-containing ligands may participate in energy transfer to the ytterbium ion, in addition to the main “antenna effect” ligand tfd. This is the first report of azobenzene group-functionalized ytterbium complexes with dual NIR luminescence and photoisomerization properties, indicating that azobenzene-containing lanthanide(III) complexes have potential applications as dual function materials in biological systems.
中文翻译:

偶氮苯基团功能化的单核和双核镱(III)配合物的近红外光致发光和可逆反式至顺式光异构化
合成并表征了具有双近红外(NIR)光致发光和可逆反式至顺式光异构化功能的两种单核和一种双核镱配合物。中心镱 (III) 离子与两个 β-二酮(4,4,4-三氟-1-苯基丁烷-1,3-二酮 (tfd))配体和一个去质子化的含偶氮苯的四齿配体 [( E ) -4 -(苯基二氮烯基)-N , N-双(吡啶-2-基甲基)苯甲酰肼(HL),( E )-4-((4-(二甲氨基)苯基)二氮烯基) -N , N-双(吡啶-2-)基甲基)苯甲酰肼(HNL),或(E)-4,4'- N ',N'-双(吡啶-2-基甲基)苯甲酰肼偶氮苯(H 2 DL)]形成中性三元络合物([Yb(tfd) ) 2 L]、[Yb(tfd) 2 (NL)] 或 [Yb 2 (tfd) 4 (DL)],分别),其中镱 (III) 离子与 N 3 O 5供体组进行八配位。X射线晶体学分析表明,所有三种配合物均形成三角十二面体几何形状,具有相似的-N=N-距离,略长于纯含偶氮苯配体的距离。在室温下,在乙醇和乙腈溶液中,在约 980 nm 的波长下测定了 Yb(III) 配合物的近红外发光特性,量子产率在 0.4-0.6% 范围内,并通过以下方法测定了反式到顺式的光异构化:量子产率 (Φ t→c = 10 –2 ) 与纯配体处于同一水平。配合物的反式光异构化速率(10 –4 s –1)略高于纯配体的光异构化速率,与偶氮苯相似(10 –5至10 –4 s –1)。根据配体第一激发三重态能级的时变密度泛函理论计算,所有配体的最低激发三重态能量均高于Yb 3+ ( 2F 5/2,1.2722 eV)。我们认为,除了主要的“天线效应”配体tfd之外,这些含偶氮配体可能还参与到镱离子的能量转移。这是首次报道具有双重近红外发光和光异构化特性的偶氮苯基功能化镱配合物,表明含偶氮苯的镧系(III)配合物作为双功能材料在生物系统中具有潜在的应用前景。
更新日期:2018-05-21
中文翻译:

偶氮苯基团功能化的单核和双核镱(III)配合物的近红外光致发光和可逆反式至顺式光异构化
合成并表征了具有双近红外(NIR)光致发光和可逆反式至顺式光异构化功能的两种单核和一种双核镱配合物。中心镱 (III) 离子与两个 β-二酮(4,4,4-三氟-1-苯基丁烷-1,3-二酮 (tfd))配体和一个去质子化的含偶氮苯的四齿配体 [( E ) -4 -(苯基二氮烯基)-N , N-双(吡啶-2-基甲基)苯甲酰肼(HL),( E )-4-((4-(二甲氨基)苯基)二氮烯基) -N , N-双(吡啶-2-)基甲基)苯甲酰肼(HNL),或(E)-4,4'- N ',N'-双(吡啶-2-基甲基)苯甲酰肼偶氮苯(H 2 DL)]形成中性三元络合物([Yb(tfd) ) 2 L]、[Yb(tfd) 2 (NL)] 或 [Yb 2 (tfd) 4 (DL)],分别),其中镱 (III) 离子与 N 3 O 5供体组进行八配位。X射线晶体学分析表明,所有三种配合物均形成三角十二面体几何形状,具有相似的-N=N-距离,略长于纯含偶氮苯配体的距离。在室温下,在乙醇和乙腈溶液中,在约 980 nm 的波长下测定了 Yb(III) 配合物的近红外发光特性,量子产率在 0.4-0.6% 范围内,并通过以下方法测定了反式到顺式的光异构化:量子产率 (Φ t→c = 10 –2 ) 与纯配体处于同一水平。配合物的反式光异构化速率(10 –4 s –1)略高于纯配体的光异构化速率,与偶氮苯相似(10 –5至10 –4 s –1)。根据配体第一激发三重态能级的时变密度泛函理论计算,所有配体的最低激发三重态能量均高于Yb 3+ ( 2F 5/2,1.2722 eV)。我们认为,除了主要的“天线效应”配体tfd之外,这些含偶氮配体可能还参与到镱离子的能量转移。这是首次报道具有双重近红外发光和光异构化特性的偶氮苯基功能化镱配合物,表明含偶氮苯的镧系(III)配合物作为双功能材料在生物系统中具有潜在的应用前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号