当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8sc01355a Dimitri F. J. Caputo 1, 2, 3 , Carlos Arroniz 1, 2, 3 , Alexander B. Dürr 1, 2, 3 , James J. Mousseau 4, 5 , Antonia F. Stepan 4, 5, 6 , Steven J. Mansfield 1, 2, 3 , Edward A. Anderson 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8sc01355a Dimitri F. J. Caputo 1, 2, 3 , Carlos Arroniz 1, 2, 3 , Alexander B. Dürr 1, 2, 3 , James J. Mousseau 4, 5 , Antonia F. Stepan 4, 5, 6 , Steven J. Mansfield 1, 2, 3 , Edward A. Anderson 1, 2, 3
Affiliation
|
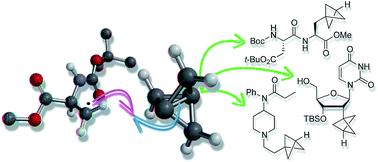
Bicyclo[1.1.1]pentanes (BCPs) are important bioisosteres of 1,4-disubstituted arenes, tert-butyl and acetylenic groups that can impart physicochemical benefits on drug candidates. Here we describe the synthesis of BCPs bearing carbon and halogen substituents under exceptionally mild reaction conditions, via triethylborane-initiated atom-transfer radical addition ring-opening of tricyclo[1.1.1.01,3]pentane (TCP) with alkyl halides. This chemistry displays broad substrate scope and functional group tolerance, enabling application to BCP analogues of biologically-relevant targets such as peptides, nucleosides, and pharmaceuticals. The BCP halide products can be converted to the parent phenyl/tert-butyl surrogates through triethylborane-promoted dehalogenation, or to other derivatives including carbonyls, alcohols, and heterocycles.
中文翻译:

高度官能化的1-卤代-3-取代的双环[1.1.1]戊烷的合成与应用†
双环[1.1.1]戊烷(BCP)是1,4-二取代的芳烃,叔丁基和炔属基团的重要生物等排体,它们可以使候选药物具有理化作用。在这里,我们通过三乙基硼烷引发的三环[1.1.1.0 1,3 ]戊烷(TCP)与烷基卤化物的三乙基硼烷引发的原子转移自由基加成开环,描述了在异常温和的反应条件下带有碳和卤素取代基的BCP的合成。这种化学方法显示出广泛的底物范围和官能团耐受性,从而能够将生物相关靶标(例如肽,核苷和药物)应用于BCP类似物。BCP卤化物可以转化为母体苯基/叔丁基通过三乙基硼烷促进的脱卤作用或其他取代基(包括羰基,醇和杂环)生成正丁基替代物。
更新日期:2018-05-21
中文翻译:

高度官能化的1-卤代-3-取代的双环[1.1.1]戊烷的合成与应用†
双环[1.1.1]戊烷(BCP)是1,4-二取代的芳烃,叔丁基和炔属基团的重要生物等排体,它们可以使候选药物具有理化作用。在这里,我们通过三乙基硼烷引发的三环[1.1.1.0 1,3 ]戊烷(TCP)与烷基卤化物的三乙基硼烷引发的原子转移自由基加成开环,描述了在异常温和的反应条件下带有碳和卤素取代基的BCP的合成。这种化学方法显示出广泛的底物范围和官能团耐受性,从而能够将生物相关靶标(例如肽,核苷和药物)应用于BCP类似物。BCP卤化物可以转化为母体苯基/叔丁基通过三乙基硼烷促进的脱卤作用或其他取代基(包括羰基,醇和杂环)生成正丁基替代物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号