Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-05-19 , DOI: 10.1016/j.bmc.2018.05.027
Li-Huai Qin , Zhi-Long Wang , Xin Xie , Ya-Qiu Long
|
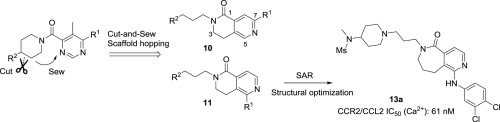
The chemokine CC receptor subtype 2 (CCR2) has attracted intensive interest for drug development in diverse therapeutic areas, including chronic inflammatory diseases, diabetes, neuropathic pain, atherogenesis and cancer. By employing a cut-and-sew scaffold hopping strategy, we identified an active scaffold of 3,4-dihydro-2,6-naphthyridin-1(2H)-one as the central pharmacophore to derive novel CCR2 antagonists. Systematic structure–activity relationship study with respect to the ring size and the substitution on the naphthyridinone ring gave birth to 1-arylamino-6-alkylheterocycle-6,7,8,9-tetrahydro-5H-pyrido[4,3-c]azepin-5-ones as a brand new chemotype of CCR2 antagonists with nanomolar inhibitory activity. The best antagonism activity in this series was exemplified by compound 13a, which combined the optimal substitutions of 3,4-dichlorophenylamino at C-1 and 3-(4-(N-methylmethylsulfonamido)piperidin-1-yl)propyl at N-6 position, leading to an IC50 value of 61 nM and 10-fold selectivity for CCR2 over CCR5. Efficient and general synthesis was established to construct the innovative core structure and derive the compound collections. This is the first report on our designed 6,7,8,9-tetrahydro-5H-pyrido[4,3-c]azepin-5-one as novel CCR2 antagonist scaffold and its synthesis.
中文翻译:

通过支架跳跃策略发现和合成6,7,8,9-四氢-5 H-吡啶并[4,3 - c ] azepin-5-one新型化学型CCR2拮抗剂
趋化因子CC受体亚型2(CCR2)在包括慢性炎症,糖尿病,神经性疼痛,动脉粥样硬化和癌症在内的多种治疗领域中引起了人们对药物开发的浓厚兴趣。通过采用切开-缝制的支架跳跃策略,我们确定了一个3,4-二氢-2,6-萘吡啶-1(2 H)-one的活性支架作为中心药效基团,以衍生出新颖的CCR2拮抗剂。关于环大小和萘啶酮环上取代的系统结构-活性关系研究产生了1-芳基氨基-6-烷基杂环-6,7,8,9-四氢-5 H-吡啶基[4,3- c] azepin-5-ones是具有纳摩尔抑制活性的新型CCR2拮抗剂的化学型。该化合物中最佳的拮抗活性以化合物13a为例,该化合物结合了C-1处的3,4-二氯苯氨基和N-6处的3-(4-(N-甲基甲基磺酰胺基)哌啶-1-基)丙基的最佳取代基位置,IC 50值为61 nM,CCR2的选择性是CCR5的10倍。建立了有效而通用的合成方法,以构建创新的核心结构并推导化合物集合。这是关于我们设计的6,7,8,9-tetrahydro-5 H -pyrido [4,3 - c ] azepin-5-one作为新型CCR2拮抗剂骨架的首次报道及其合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号