European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2018-05-19 , DOI: 10.1016/j.ejpb.2018.05.020 Luigi C. Capozzi , Roberto Pisano
|
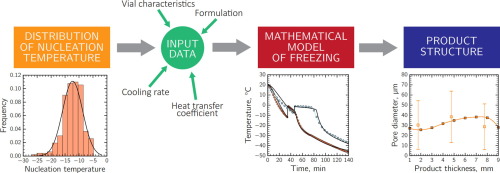
The freezing step plays a central role in reaching the most stringent requirements of quality, homogeneity and standardization of freeze-dried products. In this paper, a systematic procedure has been proposed to obtain a quantitative estimation of the pore-size variability of lyophilized products resulting from uncontrollable variations of the nucleation temperature. This procedure consisted in collecting the nucleation temperature from a statistically significant number of samples and correlating each nucleation temperature to the corresponding product morphology, using a mathematical model, to obtain a statistical description of the lyophilized product structure. This approach can also be used to obtain an estimation of the variability of the mass transfer resistance to vapor flow and, finally, of the drying time. Two different freezing configurations, i.e., conventional and suspended-vial freezing, have been used as case studies since they can produce significantly different freezing rates.
中文翻译:

寻找“黑匣子”内部:冷冻工程,以确保冷冻干燥生物制药的质量
冷冻步骤在达到冷冻干燥产品的质量,均质性和标准化最严格的要求中起着核心作用。在本文中,已经提出了一种系统的程序来定量估计由于成核温度的不可控制的变化而导致的冻干产品的孔径变化。该程序包括使用统计学模型从统计学上大量的样品中收集成核温度,并将每个成核温度与相应的产品形态相关联,以获得冻干产品结构的统计描述。该方法也可以用于获得传质阻力对蒸气流的变化以及最后的干燥时间的变化的估计。















































 京公网安备 11010802027423号
京公网安备 11010802027423号