当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,4-Diazabicyclo[2.2.2]octane-Promoted Aminotrifluoromethylthiolation of α,β-Unsaturated Carbonyl Compounds: N-Trifluoromethylthio-4-nitrophthalimide Acts as Both the Nitrogen and SCF3 Sources
Organic Letters ( IF 4.9 ) Pub Date : 2015-12-10 00:00:00 , DOI: 10.1021/acs.orglett.5b03116 Qing Xiao 1 , Qijie He 1 , Juncheng Li 1 , Jun Wang 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-12-10 00:00:00 , DOI: 10.1021/acs.orglett.5b03116 Qing Xiao 1 , Qijie He 1 , Juncheng Li 1 , Jun Wang 1
Affiliation
|
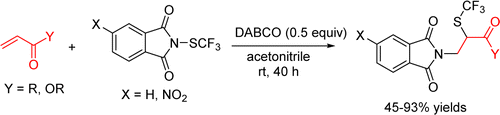
A novel difunctionalization reaction is described. It uses N-trifluoromethylthio-4-nitrophthalimide as the reagent, which serves as both the nitrogen and SCF3 sources. In the presence of DABCO (1,4-diazabicyclo[2.2.2]octane), the nitrogen and SCF3 groups can be incorporated into α,β-unsaturated carbonyl compounds easily and give versatile β-amino ketones and esters in good yields. This difunctionalization reaction features mild reaction conditions, high atom-economy, and efficient access to α-SCF3 amino acids.
中文翻译:

1,4-二氮杂双环[2.2.2]辛烷促进的α,β-不饱和羰基化合物的氨基三氟甲基硫醇化:N-三氟甲基硫基-4-硝基邻苯二甲酰亚胺同时充当氮和SCF 3的来源
描述了新的双官能化反应。它使用N-三氟甲基硫基-4-硝基邻苯二甲酰亚胺作为试剂,既用作氮源,又用作SCF 3源。在DABCO(1,4-二氮杂双环[2.2.2]辛烷)的存在下,氮和SCF 3基团可以轻松地掺入α,β-不饱和羰基化合物中,并以良好的收率得到通用的β-氨基酮和酯。此difunctionalization反应设有反应条件温和,高原子经济性,并且α-SCF高效访问3个氨基酸。
更新日期:2015-12-10
中文翻译:

1,4-二氮杂双环[2.2.2]辛烷促进的α,β-不饱和羰基化合物的氨基三氟甲基硫醇化:N-三氟甲基硫基-4-硝基邻苯二甲酰亚胺同时充当氮和SCF 3的来源
描述了新的双官能化反应。它使用N-三氟甲基硫基-4-硝基邻苯二甲酰亚胺作为试剂,既用作氮源,又用作SCF 3源。在DABCO(1,4-二氮杂双环[2.2.2]辛烷)的存在下,氮和SCF 3基团可以轻松地掺入α,β-不饱和羰基化合物中,并以良好的收率得到通用的β-氨基酮和酯。此difunctionalization反应设有反应条件温和,高原子经济性,并且α-SCF高效访问3个氨基酸。






























 京公网安备 11010802027423号
京公网安备 11010802027423号