Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.bmcl.2018.05.033 Fang Liu , Ziyou Huai , Guotai Xia , Liuping Song , Sha Li , Yulan Xu , Kangjun Hong , Mingyue Yao , Gang Liu , Yinjiu Huang
|
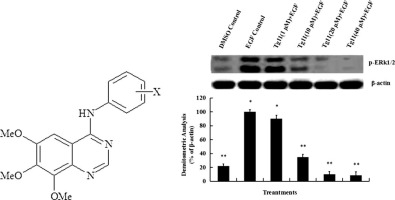
A series of 6,7,8-trimethoxy N-aryl-substituted-4-aminoquinazoline derivatives were synthesized as epidermal growth factor receptor (EGFR) inhibitors, and their antitumor activities were assessed in the gastric cancer cell line SGC7901 using MTT assay. All compounds of Tg1–14 were found to inhibit SGC7901 cell proliferation, and compound Tg11 (IC50 = 0.434 μM) was found to be slightly more effective against SGC7901 cells than epirubicin (IC50 = 5.16 μM). This suggests that compound Tg11 can be used as a new substitution structure to develop more efficacious antitumor agents. Western blot analysis showed that treatment with Tg11 (40 μM for 30 min) resulted in near complete inhibition of EGF-induced ERK1/2 phosphorylation, indicating that its anti-proliferative effect is largely associated with inhibition of ERK1/2 activation. These data imply that Tg11 is a potential anticancer agent capable of inhibiting cell proliferation.
中文翻译:

新型6,7,8-三甲氧基N-芳基取代的-4-氨基喹唑啉衍生物的合成及抗肿瘤活性
合成了一系列6,7,8-三甲氧基N-芳基取代的4-氨基喹唑啉衍生物作为表皮生长因子受体(EGFR)抑制剂,并使用MTT分析法评估了它们在胃癌细胞系SGC7901中的抗肿瘤活性。发现所有Tg1 – 14化合物均抑制SGC7901细胞增殖,并且发现化合物Tg11(IC 50 = 0.434μM)对SGC7901细胞的疗效比表柔比星(IC 50 = 5.16μM)略高。这表明化合物Tg11可以用作开发更有效的抗肿瘤剂的新的取代结构。免疫印迹分析表明用Tg11处理(40μM,持续30分钟)导致EGF诱导的ERK1 / 2磷酸化几乎完全被抑制,表明其抗增殖作用与ERK1 / 2激活的抑制密切相关。这些数据暗示Tg11是能够抑制细胞增殖的潜在抗癌剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号