当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Pathways Connecting Lead(II) Iodide and Perovskite via Polymeric Plumbate(II) Fiber
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-12-09 , DOI: 10.1021/jacs.5b10599 Yunlong Guo 1 , Kazutaka Shoyama 1 , Wataru Sato 1 , Yutaka Matsuo 1 , Kento Inoue 1 , Koji Harano 1 , Chao Liu 1 , Hideyuki Tanaka 1 , Eiichi Nakamura 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-12-09 , DOI: 10.1021/jacs.5b10599 Yunlong Guo 1 , Kazutaka Shoyama 1 , Wataru Sato 1 , Yutaka Matsuo 1 , Kento Inoue 1 , Koji Harano 1 , Chao Liu 1 , Hideyuki Tanaka 1 , Eiichi Nakamura 1
Affiliation
|
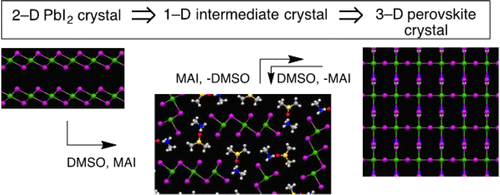
Despite tremendous progress in optoelectronic devices using lead perovskite (CH3NH3(+)PbI3(-)), there has been a paucity of mechanistic information on how photoactive micron-sized crystals of lead perovskite grow from a mixture of a layered crystal of lead(II) iodide and methylammonium iodide mediated by a polar solvent, DMSO or DMF. We report here that the whole process of the lead perovskite synthesis consists of a series of equilibria driven by reversible solvent participation involving a polymeric strip of plumbate(II) oligomer as a key intermediate. A significant finding includes quick decomposition of perovskite crystal upon exposure to DMSO or DMF at room temperature, where the solvent molecules act as a base to remove acidic ammonium iodide from the perovskite crystal. This observation accounts for the difficulty in controlling perovskite solar cell fabrication. Overall, the polar solvent is indispensible first to degrade a 2-D sheet of crystals of lead(II) iodide into 1-D fibrous intermediates and then to promote Oswald ripening of perovskite crystals. The detailed chemical information provided here will help to rationalize the photovoltaic device studies that have so far remained empirical and to open a new venue to a developing field of microscale lead perovskite devices, as illustrated by fabrication of photovoltaic devices and photodetectors.
中文翻译:

通过聚合铅酸 (II) 纤维连接碘化铅 (II) 和钙钛矿的化学途径
尽管使用铅钙钛矿 (CH3NH3(+)PbI3(-)) 的光电器件取得了巨大进展,但关于铅钙钛矿的光敏微米级晶体如何从层状铅(II ) 碘化物和甲基碘化铵,由极性溶剂、DMSO 或 DMF 介导。我们在此报告,铅钙钛矿合成的整个过程由一系列由可逆溶剂参与驱动的平衡组成,其中涉及作为关键中间体的铅酸 (II) 低聚物聚合物条。一个重要的发现包括在室温下暴露于 DMSO 或 DMF 时钙钛矿晶体的快速分解,其中溶剂分子作为碱从钙钛矿晶体中去除酸性碘化铵。这一观察结果解释了控制钙钛矿太阳能电池制造的困难。总的来说,极性溶剂对于首先将二维碘化铅 (II) 晶体片降解为一维纤维中间体,然后促进钙钛矿晶体的奥斯瓦尔德熟化是必不可少的。此处提供的详细化学信息将有助于使迄今为止仍处于经验状态的光伏器件研究合理化,并为微型铅钙钛矿器件的发展领域开辟新的领域,如光伏器件和光电探测器的制造所示。
更新日期:2015-12-09
中文翻译:

通过聚合铅酸 (II) 纤维连接碘化铅 (II) 和钙钛矿的化学途径
尽管使用铅钙钛矿 (CH3NH3(+)PbI3(-)) 的光电器件取得了巨大进展,但关于铅钙钛矿的光敏微米级晶体如何从层状铅(II ) 碘化物和甲基碘化铵,由极性溶剂、DMSO 或 DMF 介导。我们在此报告,铅钙钛矿合成的整个过程由一系列由可逆溶剂参与驱动的平衡组成,其中涉及作为关键中间体的铅酸 (II) 低聚物聚合物条。一个重要的发现包括在室温下暴露于 DMSO 或 DMF 时钙钛矿晶体的快速分解,其中溶剂分子作为碱从钙钛矿晶体中去除酸性碘化铵。这一观察结果解释了控制钙钛矿太阳能电池制造的困难。总的来说,极性溶剂对于首先将二维碘化铅 (II) 晶体片降解为一维纤维中间体,然后促进钙钛矿晶体的奥斯瓦尔德熟化是必不可少的。此处提供的详细化学信息将有助于使迄今为止仍处于经验状态的光伏器件研究合理化,并为微型铅钙钛矿器件的发展领域开辟新的领域,如光伏器件和光电探测器的制造所示。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号