当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Reactivity and Mechanism of CO2 Electroreduction at Highly Dispersed Cobalt Phthalocyanine
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acsenergylett.8b00519 Minghui Zhu 1 , Ruquan Ye 1 , Kyoungsuk Jin 1 , Nikifar Lazouski 1 , Karthish Manthiram 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acsenergylett.8b00519 Minghui Zhu 1 , Ruquan Ye 1 , Kyoungsuk Jin 1 , Nikifar Lazouski 1 , Karthish Manthiram 1
Affiliation
|
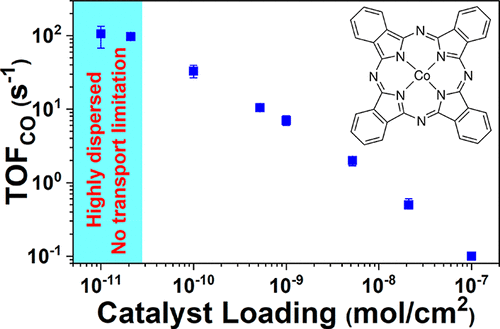
Transforming carbon dioxide to carbon monoxide with electrochemical methods allows for small-scale, modular conversion of point sources of carbon dioxide. In this work, through the preparation of a well-dispersed cobalt phthalocyanine model catalyst immobilized on carbon paper, we revealed high turnover frequencies for reducing carbon dioxide at low catalyst loadings, which are obscured at higher loadings due to aggregation. The low catalyst loadings have also enabled mechanistic studies that provide a detailed understanding of the molecular-level picture of how cobalt phthalocyanine facilitates proton and electron transfers in the rate-limiting step. We are able to tune the rate-limiting step from electron transfer to concerted proton–electron transfer, enabling higher rates of carbon dioxide reduction. Our results highlight the significance of dispersion for understanding the intrinsic catalytic performance of metal phthalocyanines for electroreduction of CO2.
中文翻译:

阐明高分散酞菁钴反应中CO 2的反应性和机理
用电化学方法将二氧化碳转化为一氧化碳可以实现二氧化碳点源的小规模模块化转化。在这项工作中,通过固定在碳纸上的分散良好的钴酞菁钴模型催化剂的制备,我们发现了在低催化剂负载量下减少二氧化碳排放的高周转频率,在较高的负载量下由于聚集而被掩盖了。低的催化剂负载量也使得能够进行机理研究,从而详细了解了钴酞菁在限速步骤中如何促进质子和电子转移的分子水平图。我们能够调整从电子转移到协调的质子-电子转移的限速步骤,从而实现更高的二氧化碳减少率。2。
更新日期:2018-05-17
中文翻译:

阐明高分散酞菁钴反应中CO 2的反应性和机理
用电化学方法将二氧化碳转化为一氧化碳可以实现二氧化碳点源的小规模模块化转化。在这项工作中,通过固定在碳纸上的分散良好的钴酞菁钴模型催化剂的制备,我们发现了在低催化剂负载量下减少二氧化碳排放的高周转频率,在较高的负载量下由于聚集而被掩盖了。低的催化剂负载量也使得能够进行机理研究,从而详细了解了钴酞菁在限速步骤中如何促进质子和电子转移的分子水平图。我们能够调整从电子转移到协调的质子-电子转移的限速步骤,从而实现更高的二氧化碳减少率。2。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号