当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.jpcb.8b01947
Alexander V. Levanov 1 , Oksana Ya. Isaikina 1 , Ulkar D. Gurbanova 1 , Valery V. Lunin 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.jpcb.8b01947
Alexander V. Levanov 1 , Oksana Ya. Isaikina 1 , Ulkar D. Gurbanova 1 , Valery V. Lunin 1
Affiliation
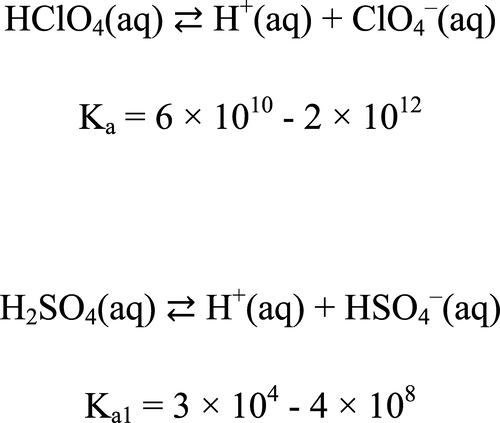
|
The experimental dissociation constants of strong acids are notoriously ill-defined, and it is necessary to rely on theoretical methods for their evaluation. We present a methodology for the theoretical evaluation of the dissociation constants, and the values of Ka for perchloric and sulfuric acids have been estimated. It has been shown that the acid dissociation constant Ka can be expressed as a product of two terms, Ka = Ka′ × fHA∞, where Ka′ is the apparent dissociation constant and fHA∞ is the infinite dilution activity coefficient of undissociated molecule of acid in liquid solution. The values of Ka′ can be computed from readily available reference data. The limiting activity coefficients fHA∞ for strong acids can be determined by theoretical methods only. The following estimate for the limiting activity coefficients of perchloric and sulfuric acids has been obtained, −2.5 < log10 fHA∞ < −1.3. The ranges of values of the dissociation constants of HClO4 and H2SO4 at 25 °C have been determined; log10 Ka(HClO4) = 10.8–12.3; log10 Ka1(H2SO4) = 4.5–8.6.
中文翻译:

高氯酸和硫酸在水溶液中的解离常数
众所周知,强酸的实验解离常数定义不清,必须依靠理论方法对其进行评估。我们提出了一种解离常数的理论评价方法,并估算了高氯酸和硫酸的K a值。已经显示的是,酸解离常数ķ一个可以被表达为两个术语的产物,ķ一个= ķ一个'× ˚F HA ∞,其中ķ一个'是表观解离常数和˚F HA ∞是液体中未解离的酸分子的无限稀释活度系数。可以从容易获得的参考数据中计算出K a '的值。限制活度系数˚F HA ∞为强酸可以仅通过理论方法来确定。已经获得的高氯酸和硫酸的限制活度系数的以下估计,-2.5 <日志10 ˚F HA ∞ <-1.3。确定了25℃下HClO 4和H 2 SO 4的解离常数的取值范围。log 10 K a(HClO 4)= 10.8–12.3; log 10 K a1(H 2 SO 4)= 4.5–8.6。
更新日期:2018-06-01
中文翻译:

高氯酸和硫酸在水溶液中的解离常数
众所周知,强酸的实验解离常数定义不清,必须依靠理论方法对其进行评估。我们提出了一种解离常数的理论评价方法,并估算了高氯酸和硫酸的K a值。已经显示的是,酸解离常数ķ一个可以被表达为两个术语的产物,ķ一个= ķ一个'× ˚F HA ∞,其中ķ一个'是表观解离常数和˚F HA ∞是液体中未解离的酸分子的无限稀释活度系数。可以从容易获得的参考数据中计算出K a '的值。限制活度系数˚F HA ∞为强酸可以仅通过理论方法来确定。已经获得的高氯酸和硫酸的限制活度系数的以下估计,-2.5 <日志10 ˚F HA ∞ <-1.3。确定了25℃下HClO 4和H 2 SO 4的解离常数的取值范围。log 10 K a(HClO 4)= 10.8–12.3; log 10 K a1(H 2 SO 4)= 4.5–8.6。

































 京公网安备 11010802027423号
京公网安备 11010802027423号