Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2018-05-17 , DOI: 10.1016/j.freeradbiomed.2018.05.063 Lindsey B Gano 1 , Li-Ping Liang 1 , Kristen Ryan 1 , Cole R Michel 1 , Joe Gomez 1 , Athanassios Vassilopoulos 2 , Nichole Reisdorph 1 , Kristofer S Fritz 1 , Manisha Patel 1
|
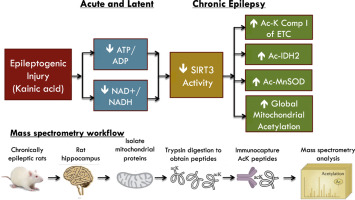
Impaired bioenergetics and oxidative damage in the mitochondria are implicated in the etiology of temporal lobe epilepsy, and hyperacetylation of mitochondrial proteins has recently emerged as a critical negative regulator of mitochondrial functions. However, the roles of mitochondrial acetylation and activity of the primary mitochondrial deacetylase, SIRT3, have not been explored in acquired epilepsy. We investigated changes in mitochondrial acetylation and SIRT3 activity in the development of chronic epilepsy in the kainic acid rat model of TLE. Hippocampal measurements were made at 48 h, 1 week and 12 weeks corresponding to the acute, latent and chronic stages of epileptogenesis. Assessment of hippocampal bioenergetics demonstrated a ≥ 27% decrease in the ATP/ADP ratio at all phases of epileptogenesis (p < 0.05), whereas cellular NAD+ levels were decreased by ≥ 41% in the acute and latent time points (p < 0.05), but not in chronically epileptic rats. In spontaneously epileptic rats, we found decreased protein expression of SIRT3 and a 60% increase in global mitochondrial acetylation, as well as enhanced acetylation of the known SIRT3 substrates MnSOD, Ndufa9 of Complex I and IDH2 (all p < 0.05), suggesting SIRT3 dysfunction in chronic epilepsy. Mass spectrometry-based acetylomics investigation of hippocampal mitochondria demonstrated a 79% increase in unique acetylated proteins from rats in the chronic phase vs. controls. Pathway analysis identified numerous mitochondrial bioenergetic pathways affected by mitochondrial acetylation. These results suggest SIRT3 dysfunction and aberrant protein acetylation may contribute to mitochondrial dysfunction in chronic epilepsy.
中文翻译:

颞叶癫痫红藻氨酸模型中线粒体乙酰化谱的改变。
线粒体生物能受损和氧化损伤与颞叶癫痫的病因有关,线粒体蛋白的过度乙酰化最近已成为线粒体功能的关键负调节因子。然而,线粒体乙酰化和主要线粒体脱乙酰酶 SIRT3 活性在获得性癫痫中的作用尚未得到探索。我们研究了红藻氨酸大鼠 TLE 模型中慢性癫痫发展过程中线粒体乙酰化和 SIRT3 活性的变化。在对应于癫痫发生的急性、潜伏和慢性阶段的 48 小时、1 周和 12 周进行海马测量。海马生物能学评估表明,在癫痫发生的所有阶段,ATP/ADP 比率均下降 ≥ 27% (p < 0.05),而细胞 NAD+ 水平在急性和潜伏时间点下降 ≥ 41% (p < 0.05),但在慢性癫痫大鼠中则不然。在自发性癫痫大鼠中,我们发现 SIRT3 蛋白表达降低,整体线粒体乙酰化增加 60%,以及已知 SIRT3 底物 MnSOD、复合物 I 的 Ndufa9 和 IDH2 的乙酰化增强(均 p < 0.05),表明 SIRT3 功能障碍在慢性癫痫中。基于质谱的海马线粒体乙酰组学研究表明,与对照组相比,慢性期大鼠的独特乙酰化蛋白增加了 79%。通路分析确定了许多受线粒体乙酰化影响的线粒体生物能通路。这些结果表明 SIRT3 功能障碍和异常蛋白乙酰化可能导致慢性癫痫中的线粒体功能障碍。

































 京公网安备 11010802027423号
京公网安备 11010802027423号