当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Harnessing Human Decellularized Blood Vessel Matrices and Cellular Construct Implants to Promote Bone Healing in an Ex Vivo Organotypic Bone Defect Model.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-05-14 , DOI: 10.1002/adhm.201800088 Stefanie Inglis 1 , Karl Heinrich Schneider 2 , Janos M Kanczler 1 , Heinz Redl 3 , Richard O C Oreffo 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-05-14 , DOI: 10.1002/adhm.201800088 Stefanie Inglis 1 , Karl Heinrich Schneider 2 , Janos M Kanczler 1 , Heinz Redl 3 , Richard O C Oreffo 1
Affiliation
|
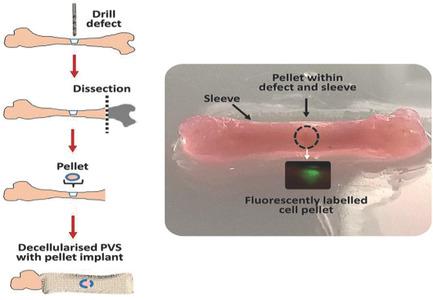
Decellularized matrices offer a beneficial substitute for biomimetic scaffolds in tissue engineering. The current study examines the potential of decellularized placental vessel sleeves (PVS) as a periosteal protective sleeve to enhance bone regeneration in embryonic day 18 chick femurs contained within the PVS and cultured organotypically over a 10 day period. The femurs are inserted into decellularized biocompatibility-tested PVS and maintained in an organotypic culture for a period of 10 days. In femurs containing decellularized PVS, a significant increase in bone volume (p < 0.001) is evident, demonstrated by microcomputed tomography (µCT) compared to femurs without PVS. Histological and immunohistological analyses reveal extensive integration of decellularized PVS with the bone periosteum, and enhanced conservation of bone architecture within the PVS. In addition, the expressions of hypoxia inducible factor-1 alpha (HIF-1α), type II collagen (COL-II), and proteoglycans are observed, indicating a possible repair mechanism via a cartilaginous stage of the bone tissue within the sleeve. The use of decellularized matrices like PVS offers a promising therapeutic strategy in surgical tissue replacement, promoting biocompatibility and architecture of the tissue as well as a factor-rich niche environment with negligible immunogenicity.
中文翻译:

利用人脱细胞的血管基质和细胞构建体植入物,以促进体内器官型骨缺损模型中的骨愈合。
去细胞基质为组织工程中的仿生支架提供了有益的替代品。目前的研究检查了脱细胞的胎盘血管套(PVS)作为骨膜保护套的潜力,以增强PVS中包含的并在器官培养10天的典型第18天鸡股骨中骨再生的能力。将股骨插入经过脱细胞生物相容性测试的PVS中,并在器官型培养物中保持10天。与没有PVS的股骨相比,通过微计算机断层扫描(µCT)可以证明,在含有脱细胞PVS的股骨中,骨体积明显增加(p <0.001)。组织学和免疫组织学分析显示,脱细胞的PVS与骨骨膜广泛整合,并增强了PVS内骨骼结构的保护性。此外,观察到缺氧诱导因子-1α(HIF-1α),II型胶原蛋白(COL-II)和蛋白聚糖的表达,表明可能通过套筒内骨组织的软骨期修复机制。像PVS这样的脱细胞基质的使用在外科手术组织置换中提供了一种有希望的治疗策略,可促进组织的生物相容性和结构以及免疫原性可忽略不计的富含因子的利基环境。
更新日期:2018-05-14
中文翻译:

利用人脱细胞的血管基质和细胞构建体植入物,以促进体内器官型骨缺损模型中的骨愈合。
去细胞基质为组织工程中的仿生支架提供了有益的替代品。目前的研究检查了脱细胞的胎盘血管套(PVS)作为骨膜保护套的潜力,以增强PVS中包含的并在器官培养10天的典型第18天鸡股骨中骨再生的能力。将股骨插入经过脱细胞生物相容性测试的PVS中,并在器官型培养物中保持10天。与没有PVS的股骨相比,通过微计算机断层扫描(µCT)可以证明,在含有脱细胞PVS的股骨中,骨体积明显增加(p <0.001)。组织学和免疫组织学分析显示,脱细胞的PVS与骨骨膜广泛整合,并增强了PVS内骨骼结构的保护性。此外,观察到缺氧诱导因子-1α(HIF-1α),II型胶原蛋白(COL-II)和蛋白聚糖的表达,表明可能通过套筒内骨组织的软骨期修复机制。像PVS这样的脱细胞基质的使用在外科手术组织置换中提供了一种有希望的治疗策略,可促进组织的生物相容性和结构以及免疫原性可忽略不计的富含因子的利基环境。















































 京公网安备 11010802027423号
京公网安备 11010802027423号