当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrazine N–N Bond Cleavage over Silica-Supported Tantalum-Hydrides
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2015-12-09 00:00:00 , DOI: 10.1021/acs.inorgchem.5b01541 Hong-Peng Jia 1 , Eric Gouré 1 , Xavier Solans-Monfort 2 , Jessica Llop Castelbou 1 , Catherine Chow 1 , Mostafa Taoufik 1 , Odile Eisenstein 3 , Elsje Alessandra Quadrelli 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2015-12-09 00:00:00 , DOI: 10.1021/acs.inorgchem.5b01541 Hong-Peng Jia 1 , Eric Gouré 1 , Xavier Solans-Monfort 2 , Jessica Llop Castelbou 1 , Catherine Chow 1 , Mostafa Taoufik 1 , Odile Eisenstein 3 , Elsje Alessandra Quadrelli 1
Affiliation
|
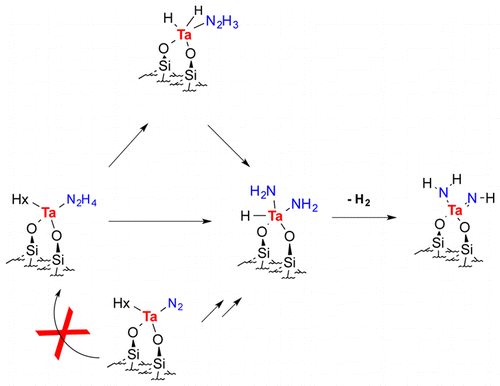
Hydrazine reacts with silica-supported tantalum-hydrides [(≡SiO)2TaHx] (x = 1, 3), 1, under mild conditions (100 °C). The IR in situ monitoring of the reaction with N2H4 or 15N2H4, and the solid-state MAS NMR spectra of the fully 15N labeled compounds (CP 15N, 1H–15N HETCOR, 1H–1H double-quantum, and 1H–1H triple-quantum spectra) were used to identify stable intermediates and products. DFT calculations were used for determining the reaction pathway and calculating the 15N and 1H NMR chemical shifts. Combining the experimental and computational studies led to the following results. At room temperature, only hydrazine adducts, 1-N2H4, are formed. Upon heating at 100 °C, the hydrazine adducts are converted to several species among which [(≡SiO)2Ta(═NH)(NH2)], 2, [(≡SiO)2TaH(NH2)2], 3, and [(≡SiO)2TaH2(NH—NH2)], 4, were identified. The final product 2 is also formed in the reaction of N2 with the same silica-supported tantalum-hydride complexes, and the species identified as 3 and 4 had been previously suggested by DFT studies as intermediates on the reaction pathway for N–N cleavage in N2. The present computational studies (cluster models with M06 functional complemented by selected calculations with periodic calculations) show that 2 is formed via 3 and 4, with either N2 or N2H4. This strengthens the previous proposal of the existence of 3 and 4 as intermediates in the reaction of N2 with the tantalum-hydrides. However, the reaction of N2 does not imply the formation of N2H4 or its hydrazido monoanionic or dianionic ligand as an intermediate. For this reason, this study informs both on the similarities and differences of the reaction pathways involving N2 and N2H4 with tantalum-hydrides.
中文翻译:

二氧化硅支撑的钽氢化物上的肼N–N键裂解
肼在温和的条件下(100°C)与氧化硅负载的氢化钽[(= SiO )2 TaH x ](x = 1,3),1反应。用N 2 H 4或15 N 2 H 4进行红外原位监测,以及全15 N标记化合物(CP 15 N,1 H– 15 N HETCOR,1 H– 1 H双量子和1 H– 1使用H三重量子光谱)鉴定稳定的中间体和产物。DFT计算用于确定反应路径并计算15 N和1 H NMR化学位移。将实验研究与计算研究相结合得出以下结果。在室温下,仅形成肼加合物1-N 2 H 4。在100°C加热后,肼加合物转化为几种化合物,其中[[ ≡SiO)2 Ta(═NH)(NH 2)],2,[(≡SiO )2 TaH(NH 2)2 ],3和[(≡SiO )2 TaH 2(NH-NH 2)],4被鉴定。最终产物2也是在N 2与相同的二氧化硅负载的氢化钽配合物的反应中形成的,DFT研究先前已将鉴定为3和4的物质作为N–N裂解反应路径的中间体在N 2中。当前的计算研究(具有M06功能的簇模型通过选定的计算与定期计算进行补充)表明2是通过3和4形成的,其中N 2或N 2 H 4。这加强了先前的建议,即在N 2与氢化钽反应中存在3和4作为中间体。但是,N的反应2并不意味着N个形成2 ħ 4或它的酰肼基的单阴离子双阴离子或配体作为中间。因此,本研究提供了涉及氢化钽的N 2和N 2 H 4的反应途径的异同。
更新日期:2015-12-09
中文翻译:

二氧化硅支撑的钽氢化物上的肼N–N键裂解
肼在温和的条件下(100°C)与氧化硅负载的氢化钽[(= SiO )2 TaH x ](x = 1,3),1反应。用N 2 H 4或15 N 2 H 4进行红外原位监测,以及全15 N标记化合物(CP 15 N,1 H– 15 N HETCOR,1 H– 1 H双量子和1 H– 1使用H三重量子光谱)鉴定稳定的中间体和产物。DFT计算用于确定反应路径并计算15 N和1 H NMR化学位移。将实验研究与计算研究相结合得出以下结果。在室温下,仅形成肼加合物1-N 2 H 4。在100°C加热后,肼加合物转化为几种化合物,其中[[ ≡SiO)2 Ta(═NH)(NH 2)],2,[(≡SiO )2 TaH(NH 2)2 ],3和[(≡SiO )2 TaH 2(NH-NH 2)],4被鉴定。最终产物2也是在N 2与相同的二氧化硅负载的氢化钽配合物的反应中形成的,DFT研究先前已将鉴定为3和4的物质作为N–N裂解反应路径的中间体在N 2中。当前的计算研究(具有M06功能的簇模型通过选定的计算与定期计算进行补充)表明2是通过3和4形成的,其中N 2或N 2 H 4。这加强了先前的建议,即在N 2与氢化钽反应中存在3和4作为中间体。但是,N的反应2并不意味着N个形成2 ħ 4或它的酰肼基的单阴离子双阴离子或配体作为中间。因此,本研究提供了涉及氢化钽的N 2和N 2 H 4的反应途径的异同。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号