当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Which Residues of the Active Site and Loop Structure of a Tyrosine Aminomutase Define Its Mutase and Lyase Activities
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1021/acs.biochem.8b00269 Gayanthi Attanayake 1 , Tyler Walter 1 , Kevin D. Walker 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1021/acs.biochem.8b00269 Gayanthi Attanayake 1 , Tyler Walter 1 , Kevin D. Walker 1, 2
Affiliation
|
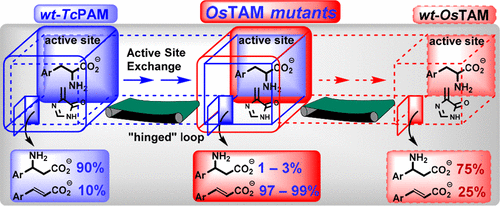
Site-directed mutations and substrate analogues were used to gain insights into the branch-point reaction of the 3,5-dihydro-5-methylidene-4H-imidazol-4-one (MIO)-tyrosine aminomutase from Oryza sativa (OsTAM). Exchanging the active residues of OsTAM (Y125C/N446K) for those in a phenylalanine aminomutase TcPAM altered its substrate specificity from tyrosine to phenylalanine. The aminomutase mechanism of OsTAM surprisingly changed almost exclusively to that of an ammonia lyase making cinnamic acid (>95%) over β-phenylalanine [Walter, T., et al. (2016) Biochemistry 55, 3497–3503]. We hypothesized that the missing electronics or sterics on the aryl ring of the phenylalanine substrate, compared with the sizable electron-donating hydroxyl of the natural tyrosine substrate, influenced the unexpected lyase reactivity of the OsTAM mutant. The double mutant was incubated with 16 α-phenylalanine substituent analogues of varying electronic strengths and sterics. The mutant converted each analogue principally to its acrylate with ∼50% conversion of the p-Br substrate, making only a small amount of the β-amino acid. The inner loop structure over the entrance to the active site was also mutated to assess how the lyase and mutase activities are affected. An OsTAM loop mutant, matching the loop residues of TcPAM, still chiefly made >95% of the acrylate from each substrate. A combined active site:loop mutant was most reactive but remained a lyase, making 10-fold more acrylates than other mutants did. While mutations within the active site changed the substrate specificity of OsTAM, continued exploration is needed to fully understand the interplay among the inner loop, the substrate, and the active site in defining the mutase and lyase activities.
中文翻译:

了解酪氨酸氨基变位酶活性位点和环结构的哪些残基定义其突变酶和裂解酶活性
定点突变和底物类似物被用于增益见解的3,5-二氢-5-亚甲基4的分支点反应ħ -咪唑-4-酮(MIO) -酪氨酸氨基变位酶从稻(锇TAM )。用Os TAM(Y125C / N446K)的活性残基交换为苯丙氨酸氨基变位酶Tc PAM中的残基,将其底物特异性从酪氨酸变为苯丙氨酸。令人惊奇的是,Os TAM的氨基变位酶机理几乎完全改变为与肉桂酸相比> 95%的肉桂酸的氨裂合酶[Walter,T.等。(2016)生物化学55,3497–3503]。我们假设苯丙氨酸底物的芳基环上缺失的电子或空间与天然酪氨酸底物的大量给电子羟基相比,会影响Os TAM突变体的意料之外的裂解酶反应性。将双突变体与具有不同电子强度和位阻的16个α-苯丙氨酸取代基类似物一起孵育。该突变体主要将每种类似物转化为其丙烯酸酯,对-Br底物的转化率约为50%,仅生成少量的β-氨基酸。活性位点入口上方的内环结构也被突变,以评估裂解酶和突变酶活性如何受到影响。一个O的TAM环突变体,匹配的环残基锝PAM仍然主要是由每种基材制成的丙烯酸酯占95%以上。组合的活性位点:环突变体反应性最强,但仍然是裂解酶,与其他突变体相比,丙烯酸酯的含量高10倍。尽管活性位点内的突变改变了Os TAM的底物特异性,但仍需要继续探索以充分了解内环,底物和活性位点之间的相互作用,从而确定突变酶和裂解酶的活性。
更新日期:2018-05-14
中文翻译:

了解酪氨酸氨基变位酶活性位点和环结构的哪些残基定义其突变酶和裂解酶活性
定点突变和底物类似物被用于增益见解的3,5-二氢-5-亚甲基4的分支点反应ħ -咪唑-4-酮(MIO) -酪氨酸氨基变位酶从稻(锇TAM )。用Os TAM(Y125C / N446K)的活性残基交换为苯丙氨酸氨基变位酶Tc PAM中的残基,将其底物特异性从酪氨酸变为苯丙氨酸。令人惊奇的是,Os TAM的氨基变位酶机理几乎完全改变为与肉桂酸相比> 95%的肉桂酸的氨裂合酶[Walter,T.等。(2016)生物化学55,3497–3503]。我们假设苯丙氨酸底物的芳基环上缺失的电子或空间与天然酪氨酸底物的大量给电子羟基相比,会影响Os TAM突变体的意料之外的裂解酶反应性。将双突变体与具有不同电子强度和位阻的16个α-苯丙氨酸取代基类似物一起孵育。该突变体主要将每种类似物转化为其丙烯酸酯,对-Br底物的转化率约为50%,仅生成少量的β-氨基酸。活性位点入口上方的内环结构也被突变,以评估裂解酶和突变酶活性如何受到影响。一个O的TAM环突变体,匹配的环残基锝PAM仍然主要是由每种基材制成的丙烯酸酯占95%以上。组合的活性位点:环突变体反应性最强,但仍然是裂解酶,与其他突变体相比,丙烯酸酯的含量高10倍。尽管活性位点内的突变改变了Os TAM的底物特异性,但仍需要继续探索以充分了解内环,底物和活性位点之间的相互作用,从而确定突变酶和裂解酶的活性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号