当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facet Dependence of CO2 Reduction Paths on Cu Electrodes
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-12-08 00:00:00 , DOI: 10.1021/acscatal.5b01967 Wenjia Luo 1 , Xiaowa Nie 2 , Michael J. Janik 3 , Aravind Asthagiri 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-12-08 00:00:00 , DOI: 10.1021/acscatal.5b01967 Wenjia Luo 1 , Xiaowa Nie 2 , Michael J. Janik 3 , Aravind Asthagiri 1
Affiliation
|
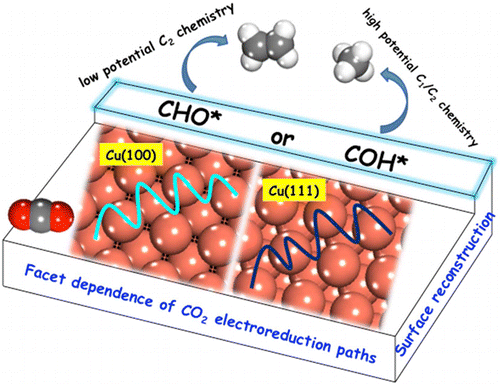
Experimental results have shown that CO2 electroreduction is sensitive to the surface morphology of Cu electrodes. We used density functional theory (DFT) to evaluate the thermodynamics and kinetics of CO2 reduction pathways on Cu(100) and Cu(111) with the aim of understanding the experimentally reported differences in CO2 reduction products. Results suggest that the hydrogenation of CO* to hydroxymethylidyne (COH*) or formyl (CHO*) is a key selective step. Cu(111) favors COH* formation, through which methane and ethylene are produced via a common CH2 species under high overpotential (<−0.8 V vs RHE). On Cu(100), formation of CHO* is preferred and ethylene formation goes through C–C coupling of two CHO* species followed by a series of reduction steps of the C2 intermediates, under relatively lower overpotential (−0.4 to −0.6 V vs RHE). Further reduction of these C2 intermediates, however, require larger potentials (∼−1.0 V vs RHE) and conflicts with the experimentally observed low potential pathway to C2 products on Cu(100). Calculations show that the presence of (111) step sites on the flat (100) terrace can reduce the overpotential for C2 production on the Cu electrode, which may be present on Cu(100) due to reconstruction. On Cu(100), a change in CO* coverage from low to high with increasing negative applied potential can trigger a switch from ethylene/ethanol to methane/ethylene as the reduction products by affecting the relative stability of CHO* and COH*.
中文翻译:

Cu电极上CO 2还原路径的切面依赖性
实验结果表明,CO 2电还原对Cu电极的表面形态敏感。我们使用密度泛函理论(DFT)评估了Cu(100)和Cu(111)上CO 2还原途径的热力学和动力学,目的是了解实验报告的CO 2还原产物的差异。结果表明,将CO *氢化为羟甲基二炔(COH *)或甲酰基(CHO *)是关键的选择性步骤。Cu(111)促进COH *的形成,通过共同的CH 2生成甲烷和乙烯高电位(<-0.8 V vs RHE)下的其他物种。在Cu(100)上,首选形成CHO *,并且乙烯形成通过两个CHO *物种的C–C偶联,然后在相对较低的过电势(-0.4至-0.6 V)下进行一系列C 2中间体的还原步骤与RHE)。然而,进一步还原这些C 2中间体需要更大的电势(相对于RHE约为-1.0 V),并且与实验观察到的向Cu(100)上C 2产物的低电势通路相冲突。计算表明,在平坦的(100)平台上存在(111)阶跃点可以减少C 2的超电势在铜电极上产生的铜,由于重建可能会存在于铜(100)上。在Cu(100)上,随着负施加电位的增加,CO *覆盖率从低到高的变化会通过影响CHO *和COH *的相对稳定性而触发乙烯/乙醇向甲烷/乙烯作为还原产物的转换。
更新日期:2015-12-08
中文翻译:

Cu电极上CO 2还原路径的切面依赖性
实验结果表明,CO 2电还原对Cu电极的表面形态敏感。我们使用密度泛函理论(DFT)评估了Cu(100)和Cu(111)上CO 2还原途径的热力学和动力学,目的是了解实验报告的CO 2还原产物的差异。结果表明,将CO *氢化为羟甲基二炔(COH *)或甲酰基(CHO *)是关键的选择性步骤。Cu(111)促进COH *的形成,通过共同的CH 2生成甲烷和乙烯高电位(<-0.8 V vs RHE)下的其他物种。在Cu(100)上,首选形成CHO *,并且乙烯形成通过两个CHO *物种的C–C偶联,然后在相对较低的过电势(-0.4至-0.6 V)下进行一系列C 2中间体的还原步骤与RHE)。然而,进一步还原这些C 2中间体需要更大的电势(相对于RHE约为-1.0 V),并且与实验观察到的向Cu(100)上C 2产物的低电势通路相冲突。计算表明,在平坦的(100)平台上存在(111)阶跃点可以减少C 2的超电势在铜电极上产生的铜,由于重建可能会存在于铜(100)上。在Cu(100)上,随着负施加电位的增加,CO *覆盖率从低到高的变化会通过影响CHO *和COH *的相对稳定性而触发乙烯/乙醇向甲烷/乙烯作为还原产物的转换。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号