当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion‐Conductive and Thermal Properties of a Synergistic Poly(ethylene carbonate)/Poly(trimethylene carbonate) Blend Electrolyte
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-05-11 , DOI: 10.1002/marc.201800146 Zhenguang Li 1 , Ronnie Mogensen 2 , Jonas Mindemark 2 , Tim Bowden 2 , Daniel Brandell 2 , Yoichi Tominaga 1
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-05-11 , DOI: 10.1002/marc.201800146 Zhenguang Li 1 , Ronnie Mogensen 2 , Jonas Mindemark 2 , Tim Bowden 2 , Daniel Brandell 2 , Yoichi Tominaga 1
Affiliation
|
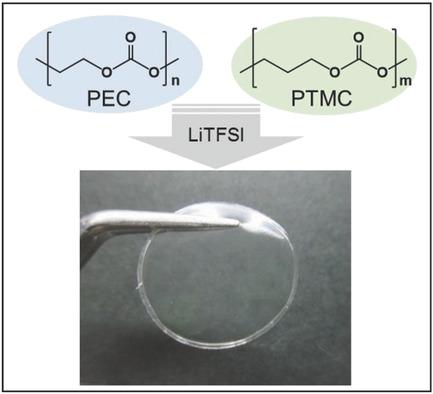
Electrolytes comprising poly(ethylene carbonate) (PEC)/poly(trimethylene carbonate) (PTM C) with lithium bis(trifluoromethane sulfonyl)imide (LiTFSI) are prepared by a simple solvent casting method. Although PEC and PTMC have similar chemical structures, they are immiscible and two glass transitions are present in the differential scanning calorimetry (DSC) measurements. Interestingly, these two polymers change to miscible blends with the addition of LiTFSI, and the ionic conductivity increases with increasing lithium salt concentration. The optimum composition of the blend electrolyte is achieved at PEC6PTMC4, with a conductivity as high as 10−6 S cm−1 at 50 °C. This value is greater than that for single PEC‐ and PTMC‐based electrolytes. Moreover, the thermal stability of the blend‐based electrolytes is improved as compared to PEC‐based electrolytes. It is clear that the interaction between CO groups and Li+ gives rise to a compatible amorphous phase of PEC and PTMC.
中文翻译:

聚碳酸亚乙酯/聚碳酸三亚甲基酯共混电解质的离子导电和热学性质
通过简单的溶剂流延法制备包含聚碳酸亚乙酯(PEC)/聚碳酸三亚甲基酯(PTM C)和双(三氟甲烷磺酰基)酰亚胺锂(LiTFSI)的电解质。尽管PEC和PTMC具有相似的化学结构,但它们是不溶混的,并且在差示扫描量热法(DSC)测量中存在两个玻璃化转变温度。有趣的是,这两种聚合物通过添加LiTFSI变成可混溶的共混物,并且离子电导率随锂盐浓度的增加而增加。混合电解质的最佳组成是在PEC 6 PTMC 4上实现的,导电率高达10 -6 S cm -1在50°C下。该值大于单个基于PEC和PTMC的电解质的值。此外,与基于PEC的电解质相比,基于共混物的电解质的热稳定性得到了改善。显然,C = O基团和Li +之间的相互作用产生了PEC和PTMC的相容的无定形相。
更新日期:2018-05-11
中文翻译:

聚碳酸亚乙酯/聚碳酸三亚甲基酯共混电解质的离子导电和热学性质
通过简单的溶剂流延法制备包含聚碳酸亚乙酯(PEC)/聚碳酸三亚甲基酯(PTM C)和双(三氟甲烷磺酰基)酰亚胺锂(LiTFSI)的电解质。尽管PEC和PTMC具有相似的化学结构,但它们是不溶混的,并且在差示扫描量热法(DSC)测量中存在两个玻璃化转变温度。有趣的是,这两种聚合物通过添加LiTFSI变成可混溶的共混物,并且离子电导率随锂盐浓度的增加而增加。混合电解质的最佳组成是在PEC 6 PTMC 4上实现的,导电率高达10 -6 S cm -1在50°C下。该值大于单个基于PEC和PTMC的电解质的值。此外,与基于PEC的电解质相比,基于共混物的电解质的热稳定性得到了改善。显然,C = O基团和Li +之间的相互作用产生了PEC和PTMC的相容的无定形相。

































 京公网安备 11010802027423号
京公网安备 11010802027423号