当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Cyclopropanol Ring Opening for Divergent Syntheses of γ-Butyrolactones and δ-Ketoesters Containing All-Carbon Quaternary Centers
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acscatal.8b00711
Zhishi Ye 1 , Xinpei Cai 1 , Jiawei Li 1 , Mingji Dai 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acscatal.8b00711
Zhishi Ye 1 , Xinpei Cai 1 , Jiawei Li 1 , Mingji Dai 1
Affiliation
|
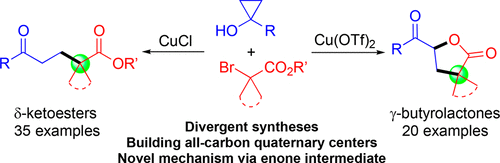
Catalytic ring opening cross coupling reactions of strained cyclopropanols have been useful for the syntheses of various β-substituted carbonyl products. Among these ring opening cross coupling reactions, the formation of α,β-unsaturated enone byproducts often competes with the desired cross coupling processes and has been a challenging synthetic problem to be addressed. Herein, we describe our efforts in developing divergent syntheses of a wide range of γ-butyrolactones and δ-ketoesters containing all-carbon quaternary centers via copper-catalyzed cyclopropanol ring opening cross couplings with 2-bromo-2,2-dialkyl esters. Our mechanistic studies reveal that unlike the previously reported cases, the formation of α,β-unsaturated enone intermediates is actually essential for the γ-butyrolactone synthesis and also contributes to the formation of the δ-ketoester product. The γ-butyrolactone synthesis is proposed to go through an intermolecular radical conjugate addition to the in situ generated α,β-unsaturated enone followed by an intramolecular radical cyclization to the ester carbonyl double bond. The reactions are effective to build all-carbon quaternary centers and have broad substrate scope.
中文翻译:

环丙醇催化开环发散合成全碳四元中心γ-丁内酯和δ-酮酯
粗环丙醇的催化开环交叉偶联反应可用于合成各种β-取代的羰基产物。在这些开环交叉偶联反应中,α,β-不饱和烯酮副产物的形成经常与所需的交叉偶联过程竞争,并且一直是一个有待解决的具有挑战性的合成问题。在此,我们描述了我们通过铜催化环丙醇开环与2-溴-2,2-二烷基酯的开环交叉偶联,开发多种含有全碳季中心的γ-丁内酯和δ-酮酯的不同合成方法。我们的机理研究表明,与之前报道的情况不同,α,β-不饱和烯酮中间体的形成实际上对于γ-丁内酯的合成至关重要,并且也有助于δ-酮酯产物的形成。 γ-丁内酯的合成建议通过分子间自由基共轭加成到原位生成的α,β-不饱和烯酮上,然后通过分子内自由基环化成酯羰基双键。该反应可有效构建全碳四元中心,并具有广泛的底物范围。
更新日期:2018-05-11
中文翻译:

环丙醇催化开环发散合成全碳四元中心γ-丁内酯和δ-酮酯
粗环丙醇的催化开环交叉偶联反应可用于合成各种β-取代的羰基产物。在这些开环交叉偶联反应中,α,β-不饱和烯酮副产物的形成经常与所需的交叉偶联过程竞争,并且一直是一个有待解决的具有挑战性的合成问题。在此,我们描述了我们通过铜催化环丙醇开环与2-溴-2,2-二烷基酯的开环交叉偶联,开发多种含有全碳季中心的γ-丁内酯和δ-酮酯的不同合成方法。我们的机理研究表明,与之前报道的情况不同,α,β-不饱和烯酮中间体的形成实际上对于γ-丁内酯的合成至关重要,并且也有助于δ-酮酯产物的形成。 γ-丁内酯的合成建议通过分子间自由基共轭加成到原位生成的α,β-不饱和烯酮上,然后通过分子内自由基环化成酯羰基双键。该反应可有效构建全碳四元中心,并具有广泛的底物范围。

































 京公网安备 11010802027423号
京公网安备 11010802027423号