当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembled Multimeric-Enzyme Nanoreactor for Robust and Efficient Biocatalysis
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00279 Liang Yin 1 , Xiang Guo 1 , Lu Liu 1 , Yong Zhang 1 , Yan Feng 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-05-10 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00279 Liang Yin 1 , Xiang Guo 1 , Lu Liu 1 , Yong Zhang 1 , Yan Feng 1
Affiliation
|
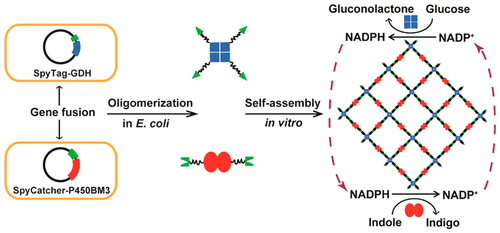
The construction of artificial multienzyme nanodevices with desired spatial arrangements have shown great promise for improving the overall performance of targeted enzyme cascades. However, it is a challenge to rationally design and construct multiple oligomeric enzyme assemblies that can be used as stable and reusable catalysts. Herein, we report a novel approach to rapidly achieve ultrastable multimeric enzyme nanoclusters (MENCs) based on enzymes property of oligomerization and the SpyTag/SpyCatcher system of orthogonally reactive split peptides. The SpyCatcher peptide and its binding partner SpyTag were fused to a dimeric cytochrome P450 monooxygenase mutant (P450BM3m) and a tetrameric glucose dehydrogenase (GDH), respectively. The fusion proteins self-assembled into the MENCs, forming a covalently coupled supramolecular multienzyme nanodevices that facilitated NADPH regeneration and converted indole into a pigment indigo. We investigated the morphology of the MENCs and found these multimeric enzymes assembled into two-dimensional layerlike nanoscale architecture, ranging from a few to several hundred square microns in size. Importantly, enzymatic analysis revealed that the MENCs not only increased the initial rate by more than three times for the indigo synthesis, but also achieved significant improvements on stability and reusability compared to unassembled enzyme mixtures. This work demonstrates a versatile and efficient strategy to construct stable and multifunctional biocatalysts with potential applications in metabolic engineering and synthetic biology.
中文翻译:

自组装的多聚酶纳米反应器,可实现强大而有效的生物催化
具有所需空间排列的人工多酶纳米装置的构建已显示出改善目标酶级联反应的整体性能的巨大希望。然而,合理设计和构建可用作稳定和可重复使用的催化剂的多种寡聚酶组装体是一个挑战。在本文中,我们报告了一种基于寡聚化酶的特性和正交反应性拆分肽段的SpyTag / SpyCatcher系统快速实现超稳定多聚酶纳米簇(MENC)的新颖方法。SpyCatcher肽及其结合伴侣SpyTag分别与二聚体细胞色素P450单加氧酶突变体(P450BM3m)和四聚体葡萄糖脱氢酶(GDH)融合。融合蛋白自组装成MENC,形成共价偶联的超分子多酶纳米器件,可促进NADPH再生并将吲哚转化为色素靛蓝。我们研究了MENC的形态,发现这些多聚酶组装成二维的层状纳米级结构,大小从几到几百平方微米不等。重要的是,酶促分析表明,与未组装的酶混合物相比,MENCs不仅使靛蓝合成的起始速率提高了三倍以上,而且在稳定性和可重复使用性方面也取得了显着改善。这项工作展示了一种构建稳定和多功能生物催化剂的通用有效策略,并在代谢工程和合成生物学中具有潜在的应用前景。
更新日期:2018-05-10
中文翻译:

自组装的多聚酶纳米反应器,可实现强大而有效的生物催化
具有所需空间排列的人工多酶纳米装置的构建已显示出改善目标酶级联反应的整体性能的巨大希望。然而,合理设计和构建可用作稳定和可重复使用的催化剂的多种寡聚酶组装体是一个挑战。在本文中,我们报告了一种基于寡聚化酶的特性和正交反应性拆分肽段的SpyTag / SpyCatcher系统快速实现超稳定多聚酶纳米簇(MENC)的新颖方法。SpyCatcher肽及其结合伴侣SpyTag分别与二聚体细胞色素P450单加氧酶突变体(P450BM3m)和四聚体葡萄糖脱氢酶(GDH)融合。融合蛋白自组装成MENC,形成共价偶联的超分子多酶纳米器件,可促进NADPH再生并将吲哚转化为色素靛蓝。我们研究了MENC的形态,发现这些多聚酶组装成二维的层状纳米级结构,大小从几到几百平方微米不等。重要的是,酶促分析表明,与未组装的酶混合物相比,MENCs不仅使靛蓝合成的起始速率提高了三倍以上,而且在稳定性和可重复使用性方面也取得了显着改善。这项工作展示了一种构建稳定和多功能生物催化剂的通用有效策略,并在代谢工程和合成生物学中具有潜在的应用前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号