当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacological Inhibition of the Ubiquitin Ligase RNF5 Rescues F508del-CFTR in Cystic Fibrosis Airway Epithelia
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-05-10 , DOI: 10.1016/j.chembiol.2018.04.010 Elvira Sondo , Federico Falchi , Emanuela Caci , Loretta Ferrera , Elisa Giacomini , Emanuela Pesce , Valeria Tomati , Sine Mandrup Bertozzi , Luca Goldoni , Andrea Armirotti , Roberto Ravazzolo , Andrea Cavalli , Nicoletta Pedemonte
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-05-10 , DOI: 10.1016/j.chembiol.2018.04.010 Elvira Sondo , Federico Falchi , Emanuela Caci , Loretta Ferrera , Elisa Giacomini , Emanuela Pesce , Valeria Tomati , Sine Mandrup Bertozzi , Luca Goldoni , Andrea Armirotti , Roberto Ravazzolo , Andrea Cavalli , Nicoletta Pedemonte
|
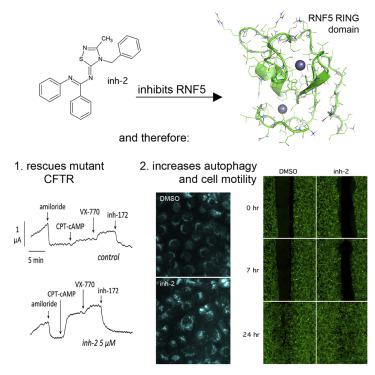
In cystic fibrosis (CF), deletion of phenylalanine 508 (F508del) in the CFTR channel is associated with misfolding and premature degradation of the mutant protein. Among the known proteins associated with F508del-CFTR processing, the ubiquitin ligase RNF5/RMA1 is particularly interesting. We previously demonstrated that genetic suppression of RNF5in vivoleads to an attenuation of intestinal pathological phenotypes in CF mice, validating the relevance of RNF5 as a drug target for CF. Here, we used a computational approach, based on ligand docking and virtual screening, to discover inh-02, a drug-like small molecule that inhibits RNF5. Inin vitroexperiments, treatment with inh-02 modulated ATG4B and paxillin, both known RNF5 targets. In immortalized and primary bronchial epithelial cells derived from CF patients homozygous for the F508del mutation, long-term incubation with inh-02 caused significant F508del-CFTR rescue. This work validates RNF5 as a drug target for CF, providing evidence to support its druggability.
中文翻译:

泛素连接酶RNF5拯救囊性纤维化气道上皮中的F508del-CFTR的药理学抑制作用
在囊性纤维化(CF)中,CFTR通道中苯丙氨酸508(F508del)的缺失与突变蛋白的错误折叠和过早降解有关。在与F508del-CFTR加工相关的已知蛋白质中,泛素连接酶RNF5 / RMA1特别令人感兴趣。我们以前证明了对RNF5的体内遗传抑制导致CF小鼠肠道病理表型的减弱,从而证实了RNF5作为CF的药物靶标的相关性。在这里,我们使用了一种基于配体对接和虚拟筛选的计算方法来发现inh-02,这是一种抑制RNF5的药物样小分子。在体外实验中,inh-02调节的ATG4B和Paxillin都是已知的RNF5靶标。在源自CF患者的F508del突变纯合的永生和原发性支气管上皮细胞中,与inh-02长期孵育可导致F508del-CFTR的大量拯救。这项工作验证了RNF5作为CF的药物靶标,为支持其可药物性提供了证据。
更新日期:2018-07-20
中文翻译:

泛素连接酶RNF5拯救囊性纤维化气道上皮中的F508del-CFTR的药理学抑制作用
在囊性纤维化(CF)中,CFTR通道中苯丙氨酸508(F508del)的缺失与突变蛋白的错误折叠和过早降解有关。在与F508del-CFTR加工相关的已知蛋白质中,泛素连接酶RNF5 / RMA1特别令人感兴趣。我们以前证明了对RNF5的体内遗传抑制导致CF小鼠肠道病理表型的减弱,从而证实了RNF5作为CF的药物靶标的相关性。在这里,我们使用了一种基于配体对接和虚拟筛选的计算方法来发现inh-02,这是一种抑制RNF5的药物样小分子。在体外实验中,inh-02调节的ATG4B和Paxillin都是已知的RNF5靶标。在源自CF患者的F508del突变纯合的永生和原发性支气管上皮细胞中,与inh-02长期孵育可导致F508del-CFTR的大量拯救。这项工作验证了RNF5作为CF的药物靶标,为支持其可药物性提供了证据。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号