当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Propargyl Radical Chemistry: Renaissance Instigated by Metal Coordination
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-03-06 00:00:00 , DOI: 10.1021/ar500365v Gagik G. Melikyan 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-03-06 00:00:00 , DOI: 10.1021/ar500365v Gagik G. Melikyan 1
Affiliation
|
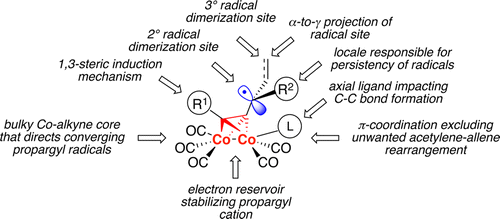
Over the last two decades, radical chemistry of propargyl systems was developed into a potent synthetic field providing access to classes of organic compounds that are otherwise hardly accessible. The levels of diastereoselection thus achieved (up to 100%) are unprecedented for free propargyl radicals, as well as for organic radicals π-bonded to transition metals. These advances were enabled by the coordination of the triple bond to a Co2(CO)6 core that precluded an acetylene–allene rearrangement, stabilized requisite propargyl cations, created conformational constraints at the carbon–carbon bond formation site, configurationally altered the acetylenic moiety allowing for 1,3-steric induction upon the newly formed stereocenters, increased bulkiness of propargyl triads thus controlling the spatial orientation of converging radicals, and allowed for α-to-γ projection of the reaction site and alteration of the transiency of radical intermediates.
中文翻译:

炔丙基自由基化学:金属配位引发的复兴
在过去的二十年中,炔丙基系统的自由基化学发展成为一个强大的合成领域,为人们提供了其他种类难以获得的有机化合物的途径。对于游离的炔丙基自由基以及π键合到过渡金属上的有机自由基,如此获得的非对映选择性水平(高达100%)是前所未有的。这些进展是通过三键与Co 2(CO)6的配位而实现的 防止乙炔-丙二烯重排的核心,稳定的必需炔丙基阳离子,在碳-碳键形成位点形成构象限制,构型改变了炔属部分,允许在新形成的立体中心上进行1,3-立体诱导,增加了炔丙基三联体的体积因此控制了聚合自由基的空间取向,并允许反应位点从α到γ投射和自由基中间体的瞬态变化。
更新日期:2015-03-06
中文翻译:

炔丙基自由基化学:金属配位引发的复兴
在过去的二十年中,炔丙基系统的自由基化学发展成为一个强大的合成领域,为人们提供了其他种类难以获得的有机化合物的途径。对于游离的炔丙基自由基以及π键合到过渡金属上的有机自由基,如此获得的非对映选择性水平(高达100%)是前所未有的。这些进展是通过三键与Co 2(CO)6的配位而实现的 防止乙炔-丙二烯重排的核心,稳定的必需炔丙基阳离子,在碳-碳键形成位点形成构象限制,构型改变了炔属部分,允许在新形成的立体中心上进行1,3-立体诱导,增加了炔丙基三联体的体积因此控制了聚合自由基的空间取向,并允许反应位点从α到γ投射和自由基中间体的瞬态变化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号