当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Metabolism of Omeprazole by Cytochrome P450 2C19 and 3A4: Mechanistic Insights from DFT Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-05-21 , DOI: 10.1021/acs.jpcb.8b01179 Kalyanashis Jana , Tusar Bandyopadhyay 1 , Bishwajit Ganguly
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-05-21 , DOI: 10.1021/acs.jpcb.8b01179 Kalyanashis Jana , Tusar Bandyopadhyay 1 , Bishwajit Ganguly
Affiliation
|
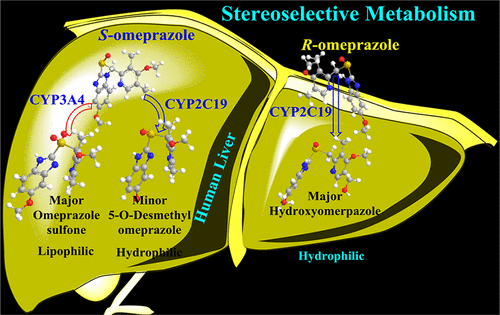
The efficacy of S-omeprazole as a proton pump inhibitor compared with that of its enantiomer R-omeprazole is studied using density functional theoretical calculations. The pharmacokinetic studies suggest that the efficacy of S-omeprazole presumably depends on metabolic pathway and excretion from the human body. The density functional theory calculations at SMDwater-B3LYP-D3/6-311+G(d,p)/LANL2DZ//B3LYP/6-31G(d)/LANL2DZ with triradicaloid model active species, [Por•+FeIV(SH)O], of CYP2C19 enzyme with high-spin quartet and low-spin doublet states demonstrate C–H bond activation mechanism through a two-state rebound process for the hydroxylation of R-omeprazole and S-omeprazole. The calculated activation free energy barriers for the hydrogen abstraction are 15.7 and 17.5 kcal/mol for R-omeprazole and S-omeprazole, respectively. The hydroxylation of R-omeprazole and S-omeprazole is thermodynamically favored; however, the hydroxylated intermediate of S-omeprazole further disintegrates to metabolite 5-O-desmethylomeprazole with a higher kinetic barrier. We have examined the sulfoxidation of S-omeprazole to omeprazole sulfone metabolite by CYP3A4, and the observed activation free energy barrier is 9.9 kcal/mol. The computational results reveal that CYP2C19 exclusively metabolizes R-omeprazole to hydroxyomeprazole, which is hydrophilic and can easily excrete, whereas CYP3A4 metabolizes S-omeprazole to lipophilic sulfone; hence, the excretion of this metabolite would be relatively slower from the body. The spin density analysis and molecular orbital analysis performed using biorthogonalization calculations indicate that R-omeprazole favors high-spin pathway for metabolism process whereas S-omeprazole prefers the low-spin pathway.
中文翻译:

通过细胞色素P450 2C19和3A4进行奥美拉唑的立体选择性代谢:DFT研究的机理性见解
使用密度泛函理论计算研究了S-奥美拉唑作为质子泵抑制剂的功效与其对映体R-奥美拉唑的功效。药代动力学研究表明,S-奥美拉唑的功效可能取决于代谢途径和人体排泄。具有三自由基模型活性物质[Por •+ Fe IV(S)的SMD水-B3LYP-D3 / 6-311 + G(d,p)/ LANL2DZ // B3LYP / 6-31G(d)/ LANL2DZ的密度泛函理论计算SH] O],具有高旋转四重态和低旋转双重态的CYP2C19酶通过两个状态的反弹过程证明C–H键激活机制使R的羟基化-奥美拉唑和S-奥美拉唑。对于R-奥美拉唑和S-奥美拉唑,计算的用于氢提取的活化自由能垒分别为15.7和17.5kcal / mol 。R-奥美拉唑和S-奥美拉唑的羟基化在热力学上是有利的;然而,S-奥美拉唑的羟基化中间体进一步分解为具有更高动力学屏障的代谢物5- O-去甲基奥美拉唑。我们已经研究了CYP3A4将S-奥美拉唑磺化氧化为奥美拉唑砜代谢产物,观察到的活化自由能垒为9.9 kcal / mol。计算结果表明CYP2C19专门代谢R-奥美拉唑为羟基奥美拉唑,后者是亲水性的,容易排泄,而CYP3A4则将S-奥美拉唑代谢为亲脂砜。因此,这种代谢产物的排泄相对较慢。使用生物正交计算进行的自旋密度分析和分子轨道分析表明,R-奥美拉唑偏爱高旋转途径进行代谢过程,而S-奥美拉唑偏爱低旋转途径。
更新日期:2018-05-22
中文翻译:

通过细胞色素P450 2C19和3A4进行奥美拉唑的立体选择性代谢:DFT研究的机理性见解
使用密度泛函理论计算研究了S-奥美拉唑作为质子泵抑制剂的功效与其对映体R-奥美拉唑的功效。药代动力学研究表明,S-奥美拉唑的功效可能取决于代谢途径和人体排泄。具有三自由基模型活性物质[Por •+ Fe IV(S)的SMD水-B3LYP-D3 / 6-311 + G(d,p)/ LANL2DZ // B3LYP / 6-31G(d)/ LANL2DZ的密度泛函理论计算SH] O],具有高旋转四重态和低旋转双重态的CYP2C19酶通过两个状态的反弹过程证明C–H键激活机制使R的羟基化-奥美拉唑和S-奥美拉唑。对于R-奥美拉唑和S-奥美拉唑,计算的用于氢提取的活化自由能垒分别为15.7和17.5kcal / mol 。R-奥美拉唑和S-奥美拉唑的羟基化在热力学上是有利的;然而,S-奥美拉唑的羟基化中间体进一步分解为具有更高动力学屏障的代谢物5- O-去甲基奥美拉唑。我们已经研究了CYP3A4将S-奥美拉唑磺化氧化为奥美拉唑砜代谢产物,观察到的活化自由能垒为9.9 kcal / mol。计算结果表明CYP2C19专门代谢R-奥美拉唑为羟基奥美拉唑,后者是亲水性的,容易排泄,而CYP3A4则将S-奥美拉唑代谢为亲脂砜。因此,这种代谢产物的排泄相对较慢。使用生物正交计算进行的自旋密度分析和分子轨道分析表明,R-奥美拉唑偏爱高旋转途径进行代谢过程,而S-奥美拉唑偏爱低旋转途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号