当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifaceted Synthetic Route to Functional Polyacrylates by Transesterification of Poly(pentafluorophenyl acrylates)
Macromolecules ( IF 5.1 ) Pub Date : 2015-12-08 00:00:00 , DOI: 10.1021/acs.macromol.5b02293 Anindita Das 1 , Patrick Theato 1
Macromolecules ( IF 5.1 ) Pub Date : 2015-12-08 00:00:00 , DOI: 10.1021/acs.macromol.5b02293 Anindita Das 1 , Patrick Theato 1
Affiliation
|
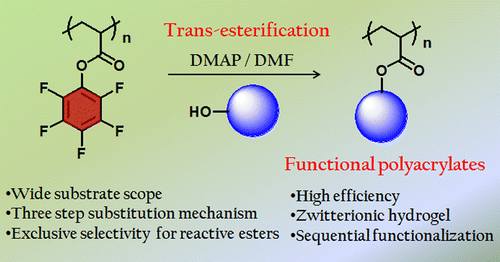
Synthesis of functional polyacrylates by 4-dimethylaminopyridine (DMAP) catalyzed trans-esterification of poly(pentafluorophenyl acrylate) (polyPFPA) is reported. High fidelity and versatility of this strategy was exemplified by near quantitative conversion with diverse functional alcohols (primary, secondary as well as phenolic) featuring reactive groups like alkene, alkyne or acrylate, enabling further sequential functionalization using click chemistry. Co-integrating an equimolar mixture of allyl and propargyl alcohol produced an orthogonally clickable copolymer by thiol–ene and 1,3-cycloaddition reaction. Base catalyzed ester exchange allowed installation of acid labile Boc-l-serine to create amino acid pendent polymer keeping both NH2- and COOH-group free, thereby providing a facile route toward zwitterionic polymers. Reaction with 2-dimethylaminoethanol conferred dual pH and CO2 responsive polymers from the same reactive precursor. The synthetic strategy was further extended to attach alcohols obtained from natural resources such as geraniol, l-lactic acid or sesamol to engineer new renewable polymers. Even a graft copolymer with very high (93%) grafting density could be achieved utilizing PEG350–OH. The trans-esterification was found to be highly selective for primary alcohols over secondary alcohols and also to the activated PFP-ester over a normal ester such as poly(methyl acrylate). Using such selectivity, fluorescently tagged polymer could be synthesized by replacing only the PFP-ester of a poly(methyl acrylate-co-PFPA) with 1-pyrenemethanol. Further, PFPA was polymerized with 2.0 mol % diacrylate to produce a cross-linked gel network. The PFP-ester groups of the cross-linked gel could be quantitatively replaced with Boc-l-serine, which upon deprotection of the Boc group resulted in a novel zwitterionic hydrogel exhibiting pH-dependent swelling properties. Time-dependent FTIR experiment suggested fast kinetics of the reaction, making this synthetic route practically applicable for postpolymerization modification. Mechanistic investigation exposed involvement of both DMAP and the nucleophilic solvent N,N-dimethylformamide (DMF) in catalyzing the reaction. This also explains the reason as to why near quantitative conversion was achieved in DMF and not in the non-nucleophilic solvent 1,4-dioxane.
中文翻译:

聚五氟苯基丙烯酸酯的酯交换反应合成功能性聚丙烯酸酯的多面合成路线
报道了通过4-二甲基氨基吡啶(DMAP)催化的聚(五氟苯基丙烯酸酯)(polyPFPA)的酯交换反应合成功能性聚丙烯酸酯。这种策略的高保真度和多功能性是通过使用具有反应性基团(如烯烃,炔烃或丙烯酸酯)的多种官能醇(伯,仲和酚)进行近乎定量的转化来举例说明的,从而可以使用点击化学进一步实现顺序官能化。将烯丙醇和炔丙醇的等摩尔混合物共混合,可通过硫醇-烯和1,3-环加成反应生成可正交点击的共聚物。碱催化的酯交换可安装酸不稳定的Boc- 1-丝氨酸,以生成氨基酸侧链聚合物,同时保留两个NH 2-和不含COOH-基团,从而提供了通往两性离子聚合物的简便途径。与2-二甲基氨基乙醇的反应使来自同一反应性前体的双重pH和CO 2响应性聚合物成为可能。合成策略进一步扩展,以连接从天然资源(如香叶醇,1-乳酸或芝麻酚)获得的醇,以工程化新的可再生聚合物。使用PEG 350甚至可以实现接枝密度很高(93%)的接枝共聚物-哦。发现酯交换反应对伯醇的反应对仲醇的选择性高,对活化的PFP-酯的反应对普通酯(如聚(丙烯酸甲酯))的选择性也高。使用这样的选择性,可以通过仅用1-py啶乙醇代替聚(丙烯酸甲酯-共-PFPA)的PFP-酯来合成荧光标记的聚合物。此外,PFPA与2.0mol%的二丙烯酸酯聚合以产生交联的凝胶网络。交联凝胶的PFP-酯基可以用Boc - l定量取代-丝氨酸,其在Boc基团上脱保护后,产生了一种新型的两性离子水凝胶,表现出pH依赖性的溶胀特性。随时间变化的FTIR实验表明,该反应具有快速动力学,因此该合成路线实际上可用于聚合后的修饰。机理研究揭示了DMAP和亲核溶剂N,N-二甲基甲酰胺(DMF)参与催化反应。这也解释了为什么在DMF中而不是在非亲核溶剂1,4-二恶烷中实现接近定量转化的原因。
更新日期:2015-12-08
中文翻译:

聚五氟苯基丙烯酸酯的酯交换反应合成功能性聚丙烯酸酯的多面合成路线
报道了通过4-二甲基氨基吡啶(DMAP)催化的聚(五氟苯基丙烯酸酯)(polyPFPA)的酯交换反应合成功能性聚丙烯酸酯。这种策略的高保真度和多功能性是通过使用具有反应性基团(如烯烃,炔烃或丙烯酸酯)的多种官能醇(伯,仲和酚)进行近乎定量的转化来举例说明的,从而可以使用点击化学进一步实现顺序官能化。将烯丙醇和炔丙醇的等摩尔混合物共混合,可通过硫醇-烯和1,3-环加成反应生成可正交点击的共聚物。碱催化的酯交换可安装酸不稳定的Boc- 1-丝氨酸,以生成氨基酸侧链聚合物,同时保留两个NH 2-和不含COOH-基团,从而提供了通往两性离子聚合物的简便途径。与2-二甲基氨基乙醇的反应使来自同一反应性前体的双重pH和CO 2响应性聚合物成为可能。合成策略进一步扩展,以连接从天然资源(如香叶醇,1-乳酸或芝麻酚)获得的醇,以工程化新的可再生聚合物。使用PEG 350甚至可以实现接枝密度很高(93%)的接枝共聚物-哦。发现酯交换反应对伯醇的反应对仲醇的选择性高,对活化的PFP-酯的反应对普通酯(如聚(丙烯酸甲酯))的选择性也高。使用这样的选择性,可以通过仅用1-py啶乙醇代替聚(丙烯酸甲酯-共-PFPA)的PFP-酯来合成荧光标记的聚合物。此外,PFPA与2.0mol%的二丙烯酸酯聚合以产生交联的凝胶网络。交联凝胶的PFP-酯基可以用Boc - l定量取代-丝氨酸,其在Boc基团上脱保护后,产生了一种新型的两性离子水凝胶,表现出pH依赖性的溶胀特性。随时间变化的FTIR实验表明,该反应具有快速动力学,因此该合成路线实际上可用于聚合后的修饰。机理研究揭示了DMAP和亲核溶剂N,N-二甲基甲酰胺(DMF)参与催化反应。这也解释了为什么在DMF中而不是在非亲核溶剂1,4-二恶烷中实现接近定量转化的原因。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号