当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvation-Driven Charge Transfer and Localization in Metal Complexes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-04-22 00:00:00 , DOI: 10.1021/ar5003939 Ariana Rondi 1 , Yuseff Rodriguez 1 , Thomas Feurer 1 , Andrea Cannizzo 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2015-04-22 00:00:00 , DOI: 10.1021/ar5003939 Ariana Rondi 1 , Yuseff Rodriguez 1 , Thomas Feurer 1 , Andrea Cannizzo 1
Affiliation
|
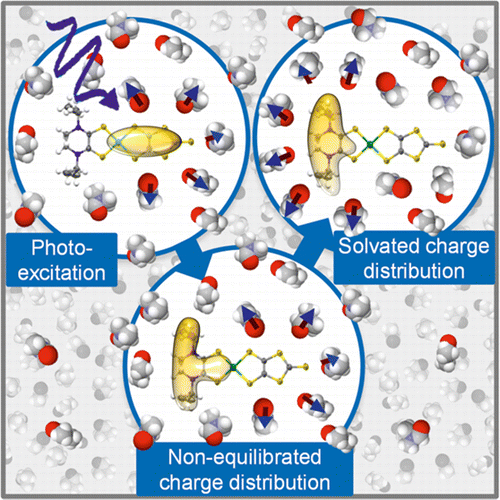
In any physicochemical process in liquids, the dynamical response of the solvent to the solutes out of equilibrium plays a crucial role in the rates and products: the solvent molecules react to the changes in volume and electron density of the solutes to minimize the free energy of the solution, thus modulating the activation barriers and stabilizing (or destabilizing) intermediate states. In charge transfer (CT) processes in polar solvents, the response of the solvent always assists the formation of charge separation states by stabilizing the energy of the localized charges. A deep understanding of the solvation mechanisms and time scales is therefore essential for a correct description of any photochemical process in dense phase and for designing molecular devices based on photosensitizers with CT excited states.
中文翻译:

金属配合物的溶剂化驱动电荷转移和局部化
在液体的任何物理化学过程中,溶剂对溶质失衡的动力学响应在速率和产物中都起着至关重要的作用:溶剂分子对溶质的体积和电子密度的变化起反应,以最大程度地降低溶质的自由能。解决方案,从而调节激活势垒并稳定(或不稳定)中间状态。在极性溶剂中的电荷转移(CT)过程中,溶剂的响应始终通过稳定局部电荷的能量来协助形成电荷分离状态。因此,深入理解溶剂化机理和时标对于正确描述稠相中的任何光化学过程以及设计基于具有CT激发态的光敏剂的分子器件至关重要。
更新日期:2015-04-22
中文翻译:

金属配合物的溶剂化驱动电荷转移和局部化
在液体的任何物理化学过程中,溶剂对溶质失衡的动力学响应在速率和产物中都起着至关重要的作用:溶剂分子对溶质的体积和电子密度的变化起反应,以最大程度地降低溶质的自由能。解决方案,从而调节激活势垒并稳定(或不稳定)中间状态。在极性溶剂中的电荷转移(CT)过程中,溶剂的响应始终通过稳定局部电荷的能量来协助形成电荷分离状态。因此,深入理解溶剂化机理和时标对于正确描述稠相中的任何光化学过程以及设计基于具有CT激发态的光敏剂的分子器件至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号