Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0075-5 Johanna R Schaub 1 , Kari A Huppert 2 , Simone N T Kurial 1, 3 , Bernadette Y Hsu 1, 3 , Ashley E Cast 2 , Bryan Donnelly 4 , Rebekah A Karns 2 , Feng Chen 1 , Milad Rezvani 1 , Hubert Y Luu 5 , Aras N Mattis 6, 7 , Anne-Laure Rougemont 8 , Philip Rosenthal 7, 9 , Stacey S Huppert 2, 10 , Holger Willenbring 1, 7, 11
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0075-5 Johanna R Schaub 1 , Kari A Huppert 2 , Simone N T Kurial 1, 3 , Bernadette Y Hsu 1, 3 , Ashley E Cast 2 , Bryan Donnelly 4 , Rebekah A Karns 2 , Feng Chen 1 , Milad Rezvani 1 , Hubert Y Luu 5 , Aras N Mattis 6, 7 , Anne-Laure Rougemont 8 , Philip Rosenthal 7, 9 , Stacey S Huppert 2, 10 , Holger Willenbring 1, 7, 11
Affiliation
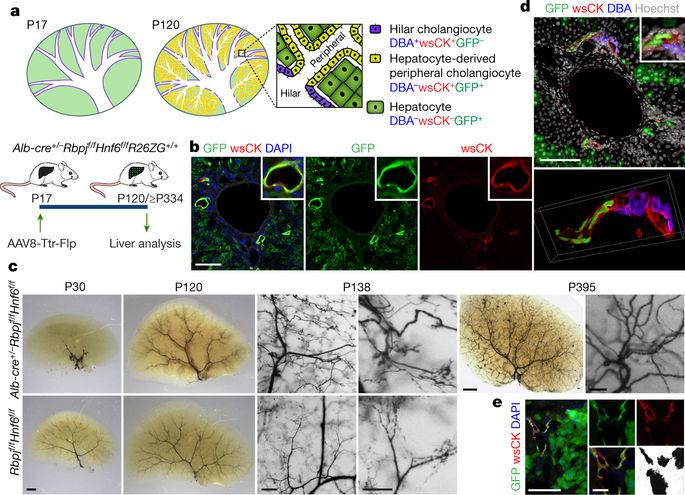
|
Transdifferentiation is a complete and stable change in cell identity that serves as an alternative to stem-cell-mediated organ regeneration. In adult mammals, findings of transdifferentiation have been limited to the replenishment of cells lost from preexisting structures, in the presence of a fully developed scaffold and niche1. Here we show that transdifferentiation of hepatocytes in the mouse liver can build a structure that failed to form in development—the biliary system in a mouse model that mimics the hepatic phenotype of human Alagille syndrome (ALGS)2. In these mice, hepatocytes convert into mature cholangiocytes and form bile ducts that are effective in draining bile and persist after the cholestatic liver injury is reversed, consistent with transdifferentiation. These findings redefine hepatocyte plasticity, which appeared to be limited to metaplasia, that is, incomplete and transient biliary differentiation as an adaptation to cell injury, based on previous studies in mice with a fully developed biliary system3–6. In contrast to bile duct development7–9, we show that de novo bile duct formation by hepatocyte transdifferentiation is independent of NOTCH signalling. We identify TGFβ signalling as the driver of this compensatory mechanism and show that it is active in some patients with ALGS. Furthermore, we show that TGFβ signalling can be targeted to enhance the formation of the biliary system from hepatocytes, and that the transdifferentiation-inducing signals and remodelling capacity of the bile-duct-deficient liver can be harnessed with transplanted hepatocytes. Our results define the regenerative potential of mammalian transdifferentiation and reveal opportunities for the treatment of ALGS and other cholestatic liver diseases.In a mouse model of a human cholestatic liver disease caused by impaired NOTCH signalling, hepatocytes transdifferentiate into cholangiocytes and form a therapeutically effective biliary system, driven by TGFβ signalling.
中文翻译:

通过 TGFβ 介导的肝细胞转分化从头形成胆道系统
转分化是细胞身份的完全和稳定变化,可作为干细胞介导的器官再生的替代方法。在成年哺乳动物中,转分化的发现仅限于在完全发育的支架和生态位存在的情况下补充从预先存在的结构中丢失的细胞。在这里,我们展示了小鼠肝脏中肝细胞的转分化可以构建一种在发育过程中未能形成的结构——小鼠模型中的胆道系统,它模仿了人类 Alagille 综合征 (ALGS)2 的肝脏表型。在这些小鼠中,肝细胞转化为成熟的胆管细胞并形成胆管,这些胆管可有效排出胆汁,并在胆汁淤积性肝损伤逆转后持续存在,与转分化一致。这些发现重新定义了肝细胞的可塑性,根据先前对具有完全发育的胆道系统的小鼠的研究,这似乎仅限于化生,即不完全和短暂的胆道分化作为对细胞损伤的适应。与胆管发育 7-9 相比,我们表明肝细胞转分化从头形成胆管与 NOTCH 信号无关。我们将 TGFβ 信号确定为这种补偿机制的驱动因素,并表明它在一些 ALGS 患者中是活跃的。此外,我们表明可以靶向 TGFβ 信号以增强肝细胞胆道系统的形成,并且可以通过移植的肝细胞利用转分化诱导信号和胆管缺陷肝脏的重塑能力。
更新日期:2018-05-01
中文翻译:

通过 TGFβ 介导的肝细胞转分化从头形成胆道系统
转分化是细胞身份的完全和稳定变化,可作为干细胞介导的器官再生的替代方法。在成年哺乳动物中,转分化的发现仅限于在完全发育的支架和生态位存在的情况下补充从预先存在的结构中丢失的细胞。在这里,我们展示了小鼠肝脏中肝细胞的转分化可以构建一种在发育过程中未能形成的结构——小鼠模型中的胆道系统,它模仿了人类 Alagille 综合征 (ALGS)2 的肝脏表型。在这些小鼠中,肝细胞转化为成熟的胆管细胞并形成胆管,这些胆管可有效排出胆汁,并在胆汁淤积性肝损伤逆转后持续存在,与转分化一致。这些发现重新定义了肝细胞的可塑性,根据先前对具有完全发育的胆道系统的小鼠的研究,这似乎仅限于化生,即不完全和短暂的胆道分化作为对细胞损伤的适应。与胆管发育 7-9 相比,我们表明肝细胞转分化从头形成胆管与 NOTCH 信号无关。我们将 TGFβ 信号确定为这种补偿机制的驱动因素,并表明它在一些 ALGS 患者中是活跃的。此外,我们表明可以靶向 TGFβ 信号以增强肝细胞胆道系统的形成,并且可以通过移植的肝细胞利用转分化诱导信号和胆管缺陷肝脏的重塑能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号