Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blastocyst-like structures generated solely from stem cells
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0051-0 Nicolas C Rivron 1, 2 , Javier Frias-Aldeguer 1, 2 , Erik J Vrij 1 , Jean-Charles Boisset 2 , Jeroen Korving 2 , Judith Vivié 2, 3 , Roman K Truckenmüller 1 , Alexander van Oudenaarden 2 , Clemens A van Blitterswijk 1 , Niels Geijsen 2, 4
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0051-0 Nicolas C Rivron 1, 2 , Javier Frias-Aldeguer 1, 2 , Erik J Vrij 1 , Jean-Charles Boisset 2 , Jeroen Korving 2 , Judith Vivié 2, 3 , Roman K Truckenmüller 1 , Alexander van Oudenaarden 2 , Clemens A van Blitterswijk 1 , Niels Geijsen 2, 4
Affiliation
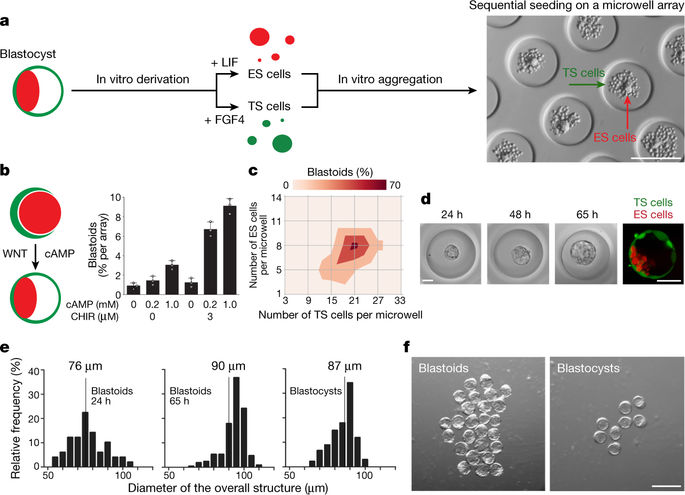
|
The blastocyst (the early mammalian embryo) forms all embryonic and extra-embryonic tissues, including the placenta. It consists of a spherical thin-walled layer, known as the trophectoderm, that surrounds a fluid-filled cavity sheltering the embryonic cells1. From mouse blastocysts, it is possible to derive both trophoblast2 and embryonic stem-cell lines3, which are in vitro analogues of the trophectoderm and embryonic compartments, respectively. Here we report that trophoblast and embryonic stem cells cooperate in vitro to form structures that morphologically and transcriptionally resemble embryonic day 3.5 blastocysts, termed blastoids. Like blastocysts, blastoids form from inductive signals that originate from the inner embryonic cells and drive the development of the outer trophectoderm. The nature and function of these signals have been largely unexplored. Genetically and physically uncoupling the embryonic and trophectoderm compartments, along with single-cell transcriptomics, reveals the extensive inventory of embryonic inductions. We specifically show that the embryonic cells maintain trophoblast proliferation and self-renewal, while fine-tuning trophoblast epithelial morphogenesis in part via a BMP4/Nodal–KLF6 axis. Although blastoids do not support the development of bona fide embryos, we demonstrate that embryonic inductions are crucial to form a trophectoderm state that robustly implants and triggers decidualization in utero. Thus, at this stage, the nascent embryo fuels trophectoderm development and implantation.Trophoblast and embryonic stem cells interact in vitro to form structures that resemble early blastocysts, and the embryo provides signals that drive early trophectoderm development and implantation.
中文翻译:

完全由干细胞产生的囊胚样结构
囊胚(早期哺乳动物胚胎)形成所有胚胎和胚胎外组织,包括胎盘。它由一个球形薄壁层组成,称为滋养外胚层,它围绕着一个充满液体的腔,为胚胎细胞提供庇护。从小鼠囊胚中,可以同时获得滋养层 2 和胚胎干细胞系 3,它们分别是滋养外胚层和胚胎区室的体外类似物。在这里我们报告滋养层细胞和胚胎干细胞在体外合作形成在形态和转录上类似于胚胎第 3.5 天囊胚的结构,称为胚泡。与囊胚一样,胚泡由源自内部胚胎细胞并驱动外部滋养外胚层发育的诱导信号形成。这些信号的性质和功能在很大程度上尚未得到探索。从遗传和物理上分离胚胎和滋养外胚层区室,以及单细胞转录组学,揭示了胚胎诱导的广泛库存。我们特别表明胚胎细胞维持滋养细胞增殖和自我更新,同时部分通过 BMP4/Nodal-KLF6 轴微调滋养细胞上皮形态发生。尽管胚状体不支持真正胚胎的发育,但我们证明胚胎诱导对于形成滋养外胚层状态至关重要,该状态在子宫内牢固植入并触发蜕膜化。因此,在这个阶段,新生胚胎为滋养外胚层的发育和植入提供燃料。滋养细胞和胚胎干细胞在体外相互作用,形成类似于早期囊胚的结构,
更新日期:2018-05-01
中文翻译:

完全由干细胞产生的囊胚样结构
囊胚(早期哺乳动物胚胎)形成所有胚胎和胚胎外组织,包括胎盘。它由一个球形薄壁层组成,称为滋养外胚层,它围绕着一个充满液体的腔,为胚胎细胞提供庇护。从小鼠囊胚中,可以同时获得滋养层 2 和胚胎干细胞系 3,它们分别是滋养外胚层和胚胎区室的体外类似物。在这里我们报告滋养层细胞和胚胎干细胞在体外合作形成在形态和转录上类似于胚胎第 3.5 天囊胚的结构,称为胚泡。与囊胚一样,胚泡由源自内部胚胎细胞并驱动外部滋养外胚层发育的诱导信号形成。这些信号的性质和功能在很大程度上尚未得到探索。从遗传和物理上分离胚胎和滋养外胚层区室,以及单细胞转录组学,揭示了胚胎诱导的广泛库存。我们特别表明胚胎细胞维持滋养细胞增殖和自我更新,同时部分通过 BMP4/Nodal-KLF6 轴微调滋养细胞上皮形态发生。尽管胚状体不支持真正胚胎的发育,但我们证明胚胎诱导对于形成滋养外胚层状态至关重要,该状态在子宫内牢固植入并触发蜕膜化。因此,在这个阶段,新生胚胎为滋养外胚层的发育和植入提供燃料。滋养细胞和胚胎干细胞在体外相互作用,形成类似于早期囊胚的结构,






























 京公网安备 11010802027423号
京公网安备 11010802027423号