当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Buildup of the Solid Electrolyte Interphase on Lithium-Metal Anodes: Reactive Molecular Dynamics Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1021/acs.jpcc.8b03046 Samuel Bertolini , Perla B. Balbuena
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1021/acs.jpcc.8b03046 Samuel Bertolini , Perla B. Balbuena
|
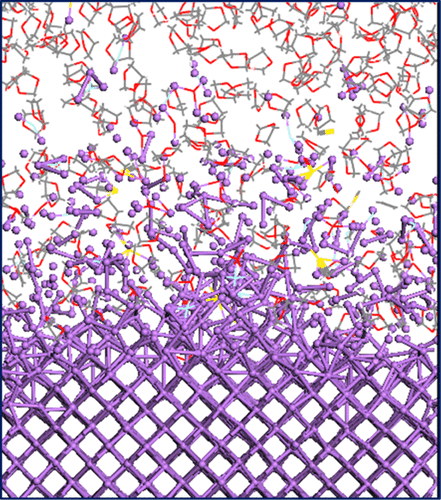
Using reactive molecular dynamics simulations, we evaluate atomistic-level interactions leading to the formation of surface films on a Li-metal (100) surface in contact with an electrolyte solution. We observe the evolution of the interfacial region and the formation of well-defined regions with varying density and oxidation state of Li; the penetration of electrolyte molecules and in some cases their electron transfer-driven decomposition leading to the initial formation of solid electrolyte interphase products. The simulations are done in the absence of a bias potential and using various electrolyte compositions including highly reactive solvents such as ethylene carbonate and less reactive solvents such as 1,3-dioxolane mixed with a 1 M concentration of a lithium salt. The structure and oxidation state of Li and some of the fragments are followed through the metal dissolution process. The results are important to understand the nature of the Li-metal anode/electrolyte interface at open-circuit potential.
中文翻译:

锂金属阳极上固体电解质中间相的建立:反应性分子动力学研究
使用反应分子动力学模拟,我们评估原子级相互作用导致与电解质溶液接触的锂金属(100)表面上形成表面膜。我们观察到界面区域的演变以及锂的密度和氧化态变化的明确区域的形成;电解质分子的渗透以及在某些情况下它们由电子传输驱动的分解导致固体电解质中间产物的初步形成。在没有偏置电位的情况下进行模拟,并使用各种电解质组合物,包括高反应性溶剂(如碳酸亚乙酯)和低反应性溶剂(如1,3-二氧戊环)与1M浓度的锂盐混合。通过金属溶解过程来追踪Li和一些碎片的结构和氧化态。该结果对于理解开路电势下锂金属阳极/电解质界面的性质非常重要。
更新日期:2018-05-02
中文翻译:

锂金属阳极上固体电解质中间相的建立:反应性分子动力学研究
使用反应分子动力学模拟,我们评估原子级相互作用导致与电解质溶液接触的锂金属(100)表面上形成表面膜。我们观察到界面区域的演变以及锂的密度和氧化态变化的明确区域的形成;电解质分子的渗透以及在某些情况下它们由电子传输驱动的分解导致固体电解质中间产物的初步形成。在没有偏置电位的情况下进行模拟,并使用各种电解质组合物,包括高反应性溶剂(如碳酸亚乙酯)和低反应性溶剂(如1,3-二氧戊环)与1M浓度的锂盐混合。通过金属溶解过程来追踪Li和一些碎片的结构和氧化态。该结果对于理解开路电势下锂金属阳极/电解质界面的性质非常重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号