PLOS ONE ( IF 2.9 ) Pub Date : 2018-05-02 , DOI: 10.1371/journal.pone.0196854 Nadja Engel , Anna Adamus , Marcus Frank , Karin Kraft , Juliane Kühn , Petra Müller , Barbara Nebe , Annika Kasten , Guido Seitz
|
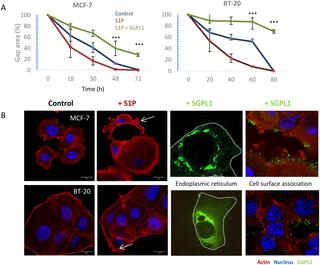
The bioactive lipid sphingosine-1-phosphate (S1P) is a main regulator of cell survival, proliferation, motility, and platelet aggregation, and it is essential for angiogenesis and lymphocyte trafficking. In that S1P acts as a second messenger intra- and extracellularly, it might promote cancer progression. The main cause is found in the high S1P concentration in the blood, which encourage cancer cells to migrate through the endothelial barrier into the blood vessels. The irreversible degradation of S1P is solely caused by the sphingosine-1-phosphate lyase (SGPL1). SGPL1 overexpression reduces cancer cell migration and therefore silences the endogenous S1P siren, which promotes cancer cell attraction—the main reason for metastasis. Since our previous metabolomics studies revealed an increased SGPL1 activity in association with successful breast cancer cell treatment in vitro, we further investigated expression and localization of SGPL1. Expression analyses confirmed a very low SGPL1 expression in all breast cancer samples, regardless of their subtype. Additionally, we were able to prove a novel SGPL expression in the cytoplasm membrane of non-tumorigenic breast cells by fusing three independent methods. The general SGPL1 downregulation and the loss of the plasma membrane expression resulted in S1P dependent stimulation of migration in the breast cancer cell lines MCF-7 and BT-20. Not only S1P stimulated migration could be repressed by overexpressing the natural SGPL1 variant not but also more general migratory activity was significantly reduced. Here, for the first time, we report on the SGPL1 plasma membrane location in human, non-malignant breast epithelial cell lines silencing the extracellular S1P siren in vitro, and thereby regulating pivotal cellular functions. Loss of this plasma membrane distribution as well as low SGPL1 expression levels could be a potential prognostic marker and a viable target for therapy. Therefore, the precise role of SGPL1 for cancer treatment should be evaluated.
中文翻译:

SGPL1表达在细胞膜中沉默乳腺上皮细胞中细胞外S1P警报的第一个证据
具有生物活性的1型神经鞘氨醇磷酸酯(S1P)是细胞存活,增殖,运动和血小板聚集的主要调节剂,对于血管生成和淋巴细胞运输至关重要。由于S1P充当细胞内和细胞外的第二信使,因此可能促进癌症的进展。主要原因是血液中的S1P浓度高,这会促使癌细胞通过内皮屏障迁移到血管中。S1P的不可逆降解仅由鞘氨醇-1-磷酸裂合酶(SGPL1)引起。SGPL1的过表达减少了癌细胞的迁移,因此使内源性S1P警笛沉默,这促进了癌细胞的吸引力-转移的主要原因。体外,我们进一步研究了SGPL1的表达和定位。表达分析证实,在所有乳腺癌样品中,无论其亚型如何,其SGPL1表达均非常低。此外,我们能够通过融合三种独立的方法在非致瘤性乳腺癌细胞的细胞质膜中证明一种新的SGPL表达。SGPL1的一般下调和质膜表达的丧失导致乳腺癌细胞MCF-7和BT-20中S1P依赖性迁移的刺激。不仅可以通过过度表达天然SGPL1变体来抑制S1P刺激的迁移,而且显着降低了更普遍的迁徙活动。在这里,我们首次报道了沉默人非恶性乳腺上皮细胞系中SGPL1质膜的位置,从而沉默了细胞外S1P警笛声在体外,从而调节关键的细胞功能。这种质膜分布的丧失以及低SGPL1表达水平可能是潜在的预后标志和可行的治疗靶标。因此,应评估SGPL1在癌症治疗中的确切作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号