当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ankyrin-mediated self-protection during cell invasion by the bacterial predator Bdellovibrio bacteriovorus.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Dec-02 , DOI: 10.1038/ncomms9884 Carey Lambert 1 , Ian T Cadby 2 , Rob Till 1 , Nhat Khai Bui 3 , Thomas R Lerner 1 , William S Hughes 2 , David J Lee 2 , Luke J Alderwick 2 , Waldemar Vollmer 3 , R Elizabeth Sockett 1 , Elizabeth R Sockett 1 , Andrew L Lovering 2
Nature Communications ( IF 14.7 ) Pub Date : 2015-Dec-02 , DOI: 10.1038/ncomms9884 Carey Lambert 1 , Ian T Cadby 2 , Rob Till 1 , Nhat Khai Bui 3 , Thomas R Lerner 1 , William S Hughes 2 , David J Lee 2 , Luke J Alderwick 2 , Waldemar Vollmer 3 , R Elizabeth Sockett 1 , Elizabeth R Sockett 1 , Andrew L Lovering 2
Affiliation
|
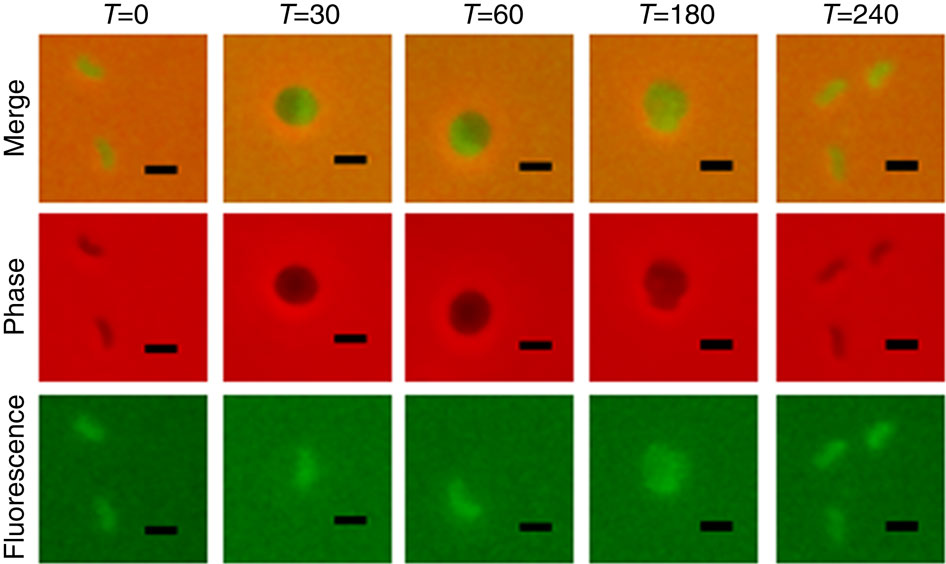
Predatory Bdellovibrio bacteriovorus are natural antimicrobial organisms, killing other bacteria by whole-cell invasion. Self-protection against prey-metabolizing enzymes is important for the evolution of predation. Initial prey entry involves the predator's peptidoglycan DD-endopeptidases, which decrosslink cell walls and prevent wasteful entry by a second predator. Here we identify and characterize a self-protection protein from B. bacteriovorus, Bd3460, which displays an ankyrin-based fold common to intracellular pathogens of eukaryotes. Co-crystal structures reveal Bd3460 complexation of dual targets, binding a conserved epitope of each of the Bd3459 and Bd0816 endopeptidases. Complexation inhibits endopeptidase activity and cell wall decrosslinking in vitro. Self-protection is vital - ΔBd3460 Bdellovibrio deleteriously decrosslink self-peptidoglycan upon invasion, adopt a round morphology, and lose predatory capacity and cellular integrity. Our analysis provides the first mechanistic examination of self-protection in Bdellovibrio, documents protection-multiplicity for products of two different genomic loci, and reveals an important evolutionary adaptation to an invasive predatory bacterial lifestyle.
中文翻译:

细菌捕食者噬菌蛭弧菌入侵细胞期间锚蛋白介导的自我保护。
捕食性噬菌弧菌是天然抗菌生物,通过全细胞入侵杀死其他细菌。针对猎物代谢酶的自我保护对于捕食的进化很重要。最初的猎物进入涉及捕食者的肽聚糖DD-内肽酶,它可以解交联细胞壁并防止第二个捕食者浪费性地进入。在这里,我们鉴定并表征了来自食菌芽孢杆菌的自我保护蛋白 Bd3460,它显示出真核生物细胞内病原体常见的基于锚蛋白的折叠。共晶结构揭示了 Bd3460 与双靶点的复合,结合 Bd3459 和 Bd0816 内肽酶的保守表位。络合可抑制体外肽链内切酶活性和细胞壁解交联。自我保护至关重要 - ΔBd3460 蛭弧菌在入侵时会有害地解交联自身肽聚糖,采用圆形形态,并失去捕食能力和细胞完整性。我们的分析首次对蛭弧菌的自我保护进行了机制检查,记录了两个不同基因组位点产物的保护多重性,并揭示了对入侵性掠食性细菌生活方式的重要进化适应。
更新日期:2015-12-05
中文翻译:

细菌捕食者噬菌蛭弧菌入侵细胞期间锚蛋白介导的自我保护。
捕食性噬菌弧菌是天然抗菌生物,通过全细胞入侵杀死其他细菌。针对猎物代谢酶的自我保护对于捕食的进化很重要。最初的猎物进入涉及捕食者的肽聚糖DD-内肽酶,它可以解交联细胞壁并防止第二个捕食者浪费性地进入。在这里,我们鉴定并表征了来自食菌芽孢杆菌的自我保护蛋白 Bd3460,它显示出真核生物细胞内病原体常见的基于锚蛋白的折叠。共晶结构揭示了 Bd3460 与双靶点的复合,结合 Bd3459 和 Bd0816 内肽酶的保守表位。络合可抑制体外肽链内切酶活性和细胞壁解交联。自我保护至关重要 - ΔBd3460 蛭弧菌在入侵时会有害地解交联自身肽聚糖,采用圆形形态,并失去捕食能力和细胞完整性。我们的分析首次对蛭弧菌的自我保护进行了机制检查,记录了两个不同基因组位点产物的保护多重性,并揭示了对入侵性掠食性细菌生活方式的重要进化适应。















































 京公网安备 11010802027423号
京公网安备 11010802027423号