当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved HCP Reduction Using a New, All‐Synthetic Depth Filtration Media Within an Antibody Purification Process
Biotechnology Journal ( IF 3.2 ) Pub Date : 2018-05-11 , DOI: 10.1002/biot.201700771 Hoang C. Nguyen 1 , Amie L. Langland 1 , John P. Amara 2 , Michael Dullen 2 , David S. Kahn 1 , Joseph A. Costanzo 1
Biotechnology Journal ( IF 3.2 ) Pub Date : 2018-05-11 , DOI: 10.1002/biot.201700771 Hoang C. Nguyen 1 , Amie L. Langland 1 , John P. Amara 2 , Michael Dullen 2 , David S. Kahn 1 , Joseph A. Costanzo 1
Affiliation
|
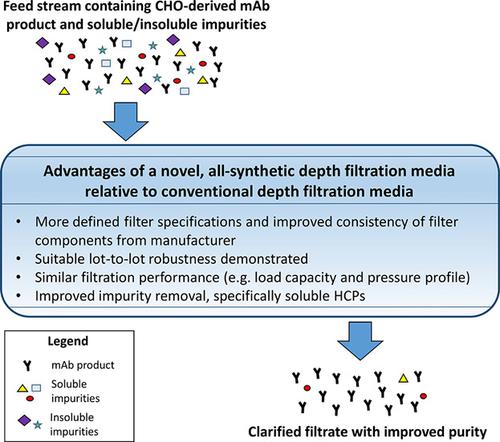
Biologic manufacturing processes typically employ clarification technologies like depth filtration to remove insoluble and soluble impurities. Conventional depth filtration media used in these processes contain naturally‐derived components like diatomaceous earth and cellulose. These components may introduce performance variability and contribute extractable/leachable components like beta‐glucans that could interfere with limulus amebocyte lysate endotoxin assays. Recently a novel, all‐synthetic depth filtration media is developed (Millistak+® HC Pro X0SP) that may improve process consistency, efficiency, and drug substance product quality by reducing soluble process impurities. This new media is evaluated against commercially available benchmark filters containing naturally‐derived components (Millistak+® HC X0HC and B1HC). Using model proteins, the synthetic media demonstrates increased binding capacity of positively charged proteins (72–126 mg g−1 media) compared to conventional media (0.3–8.6 mg g−1 media); and similar values for negatively charged species (1.3–5.6 mg g−1 media). Several CHO‐derived monoclonal antibodies (mAbs) or mAb‐like molecules are also evaluated. The X0SP filtration performance behaves similarly to benchmarks, and exhibits improved HCP reduction (at least 50% in 55% of cases tested). X0SP filtrates contained increased silicon extractables relative to benchmarks, but these were readily removed downstream. Finally, the X0SP devices demonstrates suitable lot‐to‐lot robustness when specific media components are altered intentionally to manufacturing specification limits.
中文翻译:

在抗体纯化过程中使用新型的全合成深度过滤介质改善了HCP还原
生物制造过程通常采用澄清技术,例如深度过滤,以去除不溶和可溶杂质。这些工艺中使用的常规深度过滤介质包含天然来源的成分,例如硅藻土和纤维素。这些成分可能会引入性能差异,并贡献可萃取/可浸出成分,例如β-葡聚糖,可能会干扰的变形细胞溶胞产物内毒素测定。最近一本小说,所有合成的深度过滤介质被显影(的Millistak + ® HC临X0SP),其可以通过减少可溶性过程杂质改善过程的一致性,效率和药物物质的产品质量。这项新媒体经过针对含有自然来源成分的商用基准过滤器进行了评估(Millistak +® HC X0HC和B1HC)。与常规培养基(0.3–8.6 mg g -1培养基)相比,使用模型蛋白质,合成培养基显示出带正电荷的蛋白质(72–126 mg g -1培养基)的结合能力增强;以及带负电物质的相似值(1.3–5.6 mg g -1媒体)。还评估了几种CHO衍生的单克隆抗体(mAb)或mAb样分子。X0SP过滤性能与基准性能相似,并表现出改善的HCP降低(在55%的情况下,至少降低了50%)。相对于基准,X0SP滤液中可提取的硅含量增加,但是很容易在下游去除。最后,当有意将特定的介质组件更改为制造规格限制时,X0SP设备将显示出适当的批次间鲁棒性。
更新日期:2018-09-17
中文翻译:

在抗体纯化过程中使用新型的全合成深度过滤介质改善了HCP还原
生物制造过程通常采用澄清技术,例如深度过滤,以去除不溶和可溶杂质。这些工艺中使用的常规深度过滤介质包含天然来源的成分,例如硅藻土和纤维素。这些成分可能会引入性能差异,并贡献可萃取/可浸出成分,例如β-葡聚糖,可能会干扰的变形细胞溶胞产物内毒素测定。最近一本小说,所有合成的深度过滤介质被显影(的Millistak + ® HC临X0SP),其可以通过减少可溶性过程杂质改善过程的一致性,效率和药物物质的产品质量。这项新媒体经过针对含有自然来源成分的商用基准过滤器进行了评估(Millistak +® HC X0HC和B1HC)。与常规培养基(0.3–8.6 mg g -1培养基)相比,使用模型蛋白质,合成培养基显示出带正电荷的蛋白质(72–126 mg g -1培养基)的结合能力增强;以及带负电物质的相似值(1.3–5.6 mg g -1媒体)。还评估了几种CHO衍生的单克隆抗体(mAb)或mAb样分子。X0SP过滤性能与基准性能相似,并表现出改善的HCP降低(在55%的情况下,至少降低了50%)。相对于基准,X0SP滤液中可提取的硅含量增加,但是很容易在下游去除。最后,当有意将特定的介质组件更改为制造规格限制时,X0SP设备将显示出适当的批次间鲁棒性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号