当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silylation of Aryl Halides with Monoorganosilanes Activated by Lithium Alkoxide
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1021/acs.orglett.8b00818 Takumi Yoshida 1 , Laurean Ilies 1 , Eiichi Nakamura 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1021/acs.orglett.8b00818 Takumi Yoshida 1 , Laurean Ilies 1 , Eiichi Nakamura 1
Affiliation
|
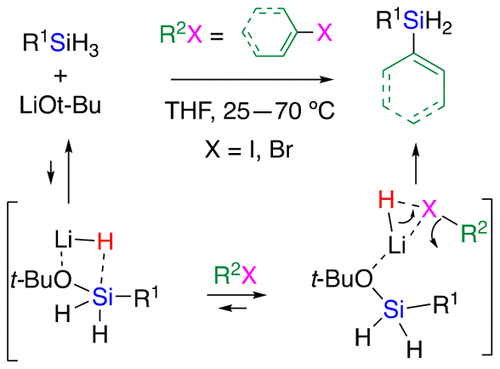
Lithium alkoxide activates a monoorganosilane to generate a transient LiH/alkoxysilane complex, which quickly reacts with aryl and alkenyl halides at 25 °C to deliver a diorganosilane product. Experimental and theoretical studies suggest that the reaction includes nucleophilic attack of LiH on the halogen atom of the organic halide to generate a transient organolithium/alkoxysilane intermediate, which undergoes quick carbon–silicon bond formation within the complex.
中文翻译:

芳基卤化物与烷氧基锂活化的单有机硅烷硅烷化
烷氧基锂激活单有机硅烷,生成瞬态LiH /烷氧基硅烷络合物,该络合物在25°C时与芳基卤化物和烯基卤化物快速反应,生成二有机硅烷产物。实验和理论研究表明,该反应包括LiH对有机卤化物的卤素原子的亲核攻击,从而生成瞬态有机锂/烷氧基硅烷中间体,该中间体在配合物中迅速形成碳-硅键。
更新日期:2018-05-01
中文翻译:

芳基卤化物与烷氧基锂活化的单有机硅烷硅烷化
烷氧基锂激活单有机硅烷,生成瞬态LiH /烷氧基硅烷络合物,该络合物在25°C时与芳基卤化物和烯基卤化物快速反应,生成二有机硅烷产物。实验和理论研究表明,该反应包括LiH对有机卤化物的卤素原子的亲核攻击,从而生成瞬态有机锂/烷氧基硅烷中间体,该中间体在配合物中迅速形成碳-硅键。































 京公网安备 11010802027423号
京公网安备 11010802027423号