当前位置:
X-MOL 学术
›
npj Vaccines
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FIV vaccine with receptor epitopes results in neutralizing antibodies but does not confer resistance to challenge.
npj Vaccines ( IF 6.9 ) Pub Date : 2018-01-01 , DOI: 10.1038/s41541-018-0051-y Craig Miller , Mauren Emanuelli , Elizabeth Fink , Esther Musselman , Ryan Mackie , Ryan Troyer , John Elder , Sue VandeWoude
npj Vaccines ( IF 6.9 ) Pub Date : 2018-01-01 , DOI: 10.1038/s41541-018-0051-y Craig Miller , Mauren Emanuelli , Elizabeth Fink , Esther Musselman , Ryan Mackie , Ryan Troyer , John Elder , Sue VandeWoude
|
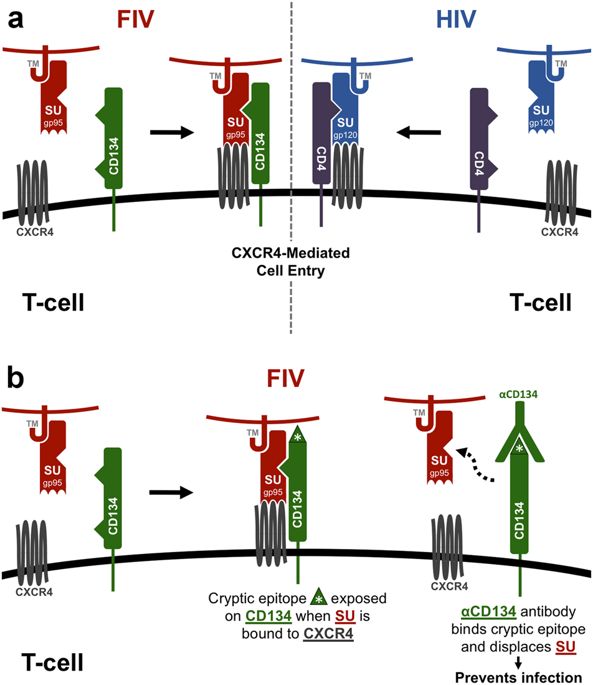
Feline immunodeficiency virus (FIV) is the feline analogue to human immunodeficiency virus (HIV) and utilizes parallel modes of receptor-mediated entry. The FIV surface glycoprotein (SU) is an important target for induction of neutralizing antibodies, and autoantibodies to the FIV binding receptor (CD134) block infection ex vivo; thus highlighting the potential for immunotherapies which utilize anti-receptor antibodies to block viral infection. To determine whether vaccination with CD134-SU complexes could induce protection against FIV infection, cats (n = 5 per group) were immunized with soluble CD134, recombinant FIV-SU protein, and/or CD134+SU complexes. Two trials were performed with different antigen combinations and vaccination schedules. In vivo generation of anti-CD134 and anti-SU IgG antibodies was measured, and in vitro neutralization assays were conducted. Immunization induced production of anti-CD134 and anti-SU antibodies that significantly inhibited FIV infection in vitro. However, no vaccine combination protected cats from FIV infection, and neat serum from vaccinated cats enhanced FIV growth in vitro. CD134+SU vaccinated cats exhibited increased CD4:CD8 ratio immediately prior to challenge, and antibodies were much more efficiently generated against vaccine by-products versus target antigens. Results suggest vaccination against viral and cryptic receptor epitopes yields neutralizing antibodies that synergistically inhibit FIV infection in vitro. Factors contributing to vaccine failure may include: (1) Heat-labile serum factors that enhance viral replication, (2) changes in circulating target cell populations induced by vaccination, and (3) weak immunogenicity of neutralizing epitopes compared to off-target vaccine components. Results reinforce the need to monitor vaccine preparation components and avoid non-specific immune stimulation during vaccination.
中文翻译:

具有受体表位的FIV疫苗产生中和抗体,但不赋予对攻击的抗性。
猫免疫缺陷病毒(FIV)是人免疫缺陷病毒(HIV)的猫类似物,并利用受体介导的进入的平行模式。FIV表面糖蛋白(SU)是诱导中和抗体的重要靶标,针对FIV结合受体(CD134)的自身抗体可阻止离体感染;因此突出了利用抗受体抗体阻断病毒感染的免疫疗法的潜力。为了确定CD134-SU复合物的疫苗接种是否可以诱导针对FIV感染的保护,猫(n 每组= 5)用可溶性CD134,重组FIV-SU蛋白和/或CD134 + SU复合物免疫。使用不同的抗原组合和疫苗接种时间表进行了两项试验。测量了体内抗CD134和抗SU IgG抗体的产生,并进行了体外中和测定。免疫诱导了抗CD134和抗SU抗体的产生,这些抗体在体外显着抑制了FIV感染。但是,没有疫苗组合可以保护猫免受FIV感染,而接种过的猫的纯净血清可以增强FIV在体外的生长。CD134 + SU疫苗接种的猫在攻击前即刻表现出增加的CD4:CD8比,与目标抗原相比,针对疫苗副产物的抗体生成效率更高。结果表明,针对病毒和隐性受体表位的疫苗接种可产生中和抗体,可在体外协同抑制FIV感染。导致疫苗失败的因素可能包括:(1)增强病毒复制的不耐热血清因素;(2)疫苗诱导的循环靶细胞群体发生变化;(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。
更新日期:2019-01-26
中文翻译:

具有受体表位的FIV疫苗产生中和抗体,但不赋予对攻击的抗性。
猫免疫缺陷病毒(FIV)是人免疫缺陷病毒(HIV)的猫类似物,并利用受体介导的进入的平行模式。FIV表面糖蛋白(SU)是诱导中和抗体的重要靶标,针对FIV结合受体(CD134)的自身抗体可阻止离体感染;因此突出了利用抗受体抗体阻断病毒感染的免疫疗法的潜力。为了确定CD134-SU复合物的疫苗接种是否可以诱导针对FIV感染的保护,猫(n 每组= 5)用可溶性CD134,重组FIV-SU蛋白和/或CD134 + SU复合物免疫。使用不同的抗原组合和疫苗接种时间表进行了两项试验。测量了体内抗CD134和抗SU IgG抗体的产生,并进行了体外中和测定。免疫诱导了抗CD134和抗SU抗体的产生,这些抗体在体外显着抑制了FIV感染。但是,没有疫苗组合可以保护猫免受FIV感染,而接种过的猫的纯净血清可以增强FIV在体外的生长。CD134 + SU疫苗接种的猫在攻击前即刻表现出增加的CD4:CD8比,与目标抗原相比,针对疫苗副产物的抗体生成效率更高。结果表明,针对病毒和隐性受体表位的疫苗接种可产生中和抗体,可在体外协同抑制FIV感染。导致疫苗失败的因素可能包括:(1)增强病毒复制的不耐热血清因素;(2)疫苗诱导的循环靶细胞群体发生变化;(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。(3)与脱靶疫苗成分相比,中和表位的免疫原性较弱。结果增加了监测疫苗制剂成分并避免在疫苗接种过程中产生非特异性免疫刺激的需要。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号