当前位置:
X-MOL 学术
›
Microb. Biotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a feruloyl esterase from Aspergillus terreus facilitates the division of fungal enzymes from Carbohydrate Esterase family 1 of the carbohydrate‐active enzymes (CAZy) database
Microbial Biotechnology ( IF 4.8 ) Pub Date : 2018-04-26 , DOI: 10.1111/1751-7915.13273 Miia R. Mäkelä 1 , Adiphol Dilokpimol 2 , Salla M. Koskela 1 , Jaana Kuuskeri 1 , Ronald P. de Vries 2 , Kristiina Hildén 1
Microbial Biotechnology ( IF 4.8 ) Pub Date : 2018-04-26 , DOI: 10.1111/1751-7915.13273 Miia R. Mäkelä 1 , Adiphol Dilokpimol 2 , Salla M. Koskela 1 , Jaana Kuuskeri 1 , Ronald P. de Vries 2 , Kristiina Hildén 1
Affiliation
|
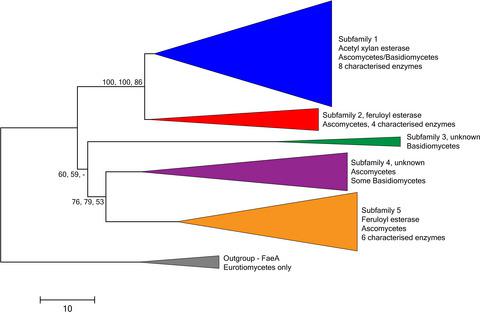
Feruloyl esterases (FAEs) are accessory enzymes for plant biomass degradation, which catalyse hydrolysis of carboxylic ester linkages between hydroxycinnamic acids and plant cell‐wall carbohydrates. They are a diverse group of enzymes evolved from, e.g. acetyl xylan esterases (AXEs), lipases and tannases, thus complicating their classification and prediction of function by sequence similarity. Recently, an increasing number of fungal FAEs have been biochemically characterized, owing to their potential in various biotechnological applications and multitude of candidate FAEs in fungal genomes. However, only part of the fungal FAEs are included in Carbohydrate Esterase family 1 (CE1) of the carbohydrate‐active enzymes (CAZy) database. In this work, we performed a phylogenetic analysis that divided the fungal members of CE1 into five subfamilies of which three contained characterized enzymes with conserved activities. Conservation within one of the subfamilies was confirmed by characterization of an additional CE1 enzyme from Aspergillus terreus. Recombinant A. terreus FaeD (AtFaeD) showed broad specificity towards synthetic methyl and ethyl esters, and released ferulic acid from plant biomass substrates, demonstrating its true FAE activity and interesting features as potential biocatalyst. The subfamily division of the fungal CE1 members enables more efficient selection of candidate enzymes for biotechnological processes.
中文翻译:

表征曲霉曲霉的阿魏酸酯酶有助于碳水化合物活性酶(CAZy)数据库的碳水化合物酯酶家族1的真菌酶的分离
阿魏酸酯酶(FAE)是植物生物量降解的辅助酶,可催化羟基肉桂酸与植物细胞壁碳水化合物之间的羧酸酯键水解。它们是从例如乙酰木聚糖酯酶(AXE),脂肪酶和鞣酸酶进化而来的多种酶,因此通过序列相似性使它们的分类和功能预测变得复杂。近来,由于其在各种生物技术应用中的潜力以及在真菌基因组中的大量候选FAE的潜力,已经对越来越多的真菌FAE进行了生物化学表征。但是,碳水化合物活性酶(CAZy)数据库的碳水化合物酯酶家族1(CE1)中仅包含部分真菌FAE。在这项工作中,我们进行了系统发育分析,将CE1的真菌成员分为五个亚家族,其中三个包含具有保守活性的特征化酶。一个亚科中的保守性通过鉴定另外一种CE1酶的特性得到了证实。曲霉曲霉。重组土曲霉FaeD(AtFaeD)显示由植物生物质底物向合成甲基和乙基酯广泛的特异性,并释放阿魏酸,表明其真实FAE活性和有趣的功能作为潜在的生物催化剂。真菌CE1成员的亚科划分使得能够更有效地选择用于生物技术过程的候选酶。
更新日期:2018-04-26
中文翻译:

表征曲霉曲霉的阿魏酸酯酶有助于碳水化合物活性酶(CAZy)数据库的碳水化合物酯酶家族1的真菌酶的分离
阿魏酸酯酶(FAE)是植物生物量降解的辅助酶,可催化羟基肉桂酸与植物细胞壁碳水化合物之间的羧酸酯键水解。它们是从例如乙酰木聚糖酯酶(AXE),脂肪酶和鞣酸酶进化而来的多种酶,因此通过序列相似性使它们的分类和功能预测变得复杂。近来,由于其在各种生物技术应用中的潜力以及在真菌基因组中的大量候选FAE的潜力,已经对越来越多的真菌FAE进行了生物化学表征。但是,碳水化合物活性酶(CAZy)数据库的碳水化合物酯酶家族1(CE1)中仅包含部分真菌FAE。在这项工作中,我们进行了系统发育分析,将CE1的真菌成员分为五个亚家族,其中三个包含具有保守活性的特征化酶。一个亚科中的保守性通过鉴定另外一种CE1酶的特性得到了证实。曲霉曲霉。重组土曲霉FaeD(AtFaeD)显示由植物生物质底物向合成甲基和乙基酯广泛的特异性,并释放阿魏酸,表明其真实FAE活性和有趣的功能作为潜在的生物催化剂。真菌CE1成员的亚科划分使得能够更有效地选择用于生物技术过程的候选酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号