当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxa‐Michael Addition to α,β‐Unsaturated Nitriles: An Expedient Route to γ‐Amino Alcohols and Derivatives
ChemCatChem ( IF 3.8 ) Pub Date : 2018-05-08 , DOI: 10.1002/cctc.201800509 Beibei Guo 1 , Douwe S Zijlstra 1 , Johannes G de Vries 1, 2 , Edwin Otten 1
ChemCatChem ( IF 3.8 ) Pub Date : 2018-05-08 , DOI: 10.1002/cctc.201800509 Beibei Guo 1 , Douwe S Zijlstra 1 , Johannes G de Vries 1, 2 , Edwin Otten 1
Affiliation
|
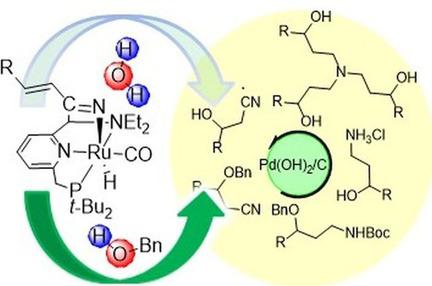
Water addition to α,β‐unsaturated nitriles would give facile access to the β‐hydroxy‐nitriles, which in turn can be hydrogenated to the γ‐amino alcohols. We have previously shown that alcohols readily add in 1,4‐fashion to these substrates using Milstein's Ru(PNN) pincer complex as catalyst. However, attempted water addition to α,β‐unsaturated nitriles gave the 3‐hydroxynitriles in mediocre yields. On the other hand, addition of benzyl alcohol proceeded in excellent yields for a variety of β‐substituted unsaturated nitriles. Subsequent treatment of the benzyl alcohol addition products with TMSCl/FeCl3 resulted in the formation of 3‐hydroxy‐alkylnitriles. The 3‐benzyloxy‐alkylnitriles obtained from oxa‐Michael addition also could be hydrogenated directly in the presence of acid to give the amino alcohols as their HCl salts in excellent yields. Hydrogenation under neutral conditions gave a mixture of the secondary and tertiary amines. Hydrogenation in the presence of base and Boc‐anhydride gave the orthogonally bis‐protected amino alcohols, in which the benzyl ether can subsequently be cleaved to yield Boc‐protected amino alcohols. Thus, a variety of molecular scaffolds with a 1,3‐relationship between O‐ and N‐functional group is accessible starting from oxa‐Michael addition of benzyl alcohol to α,β‐unsaturated nitriles.
中文翻译:

α,β-不饱和腈中的 Oxa-Michael 加成:生成 γ-氨基醇和衍生物的便捷途径
α,β-不饱和腈中加水可以很容易地得到β-羟基腈,而β-羟基腈又可以氢化成γ-氨基醇。我们之前已经证明,使用 Milstein 的 Ru(PNN) 钳配合物作为催化剂,醇很容易以 1,4-方式添加到这些底物中。然而,尝试将水添加到 α,β-不饱和腈中,得到的 3-羟基腈收率平平。另一方面,苯甲醇的加成反应对各种β-取代不饱和腈的产率都很高。随后用 TMSCl/FeCl 3处理苯甲醇加成产物,形成 3-羟基烷基腈。由 oxa-Michael 加成得到的 3-苄氧基-烷基腈也可以在酸存在下直接氢化,以优异的收率得到 HCl 盐形式的氨基醇。在中性条件下氢化得到仲胺和叔胺的混合物。在碱和 Boc 酸酐存在下氢化,得到正交双保护的氨基醇,其中苄基醚随后可裂解生成 Boc 保护的氨基醇。因此,从苯甲醇与 α,β-不饱和腈的 oxa-Michael 加成开始,可以得到各种O和N官能团之间具有 1,3-关系的分子支架。
更新日期:2018-05-08
中文翻译:

α,β-不饱和腈中的 Oxa-Michael 加成:生成 γ-氨基醇和衍生物的便捷途径
α,β-不饱和腈中加水可以很容易地得到β-羟基腈,而β-羟基腈又可以氢化成γ-氨基醇。我们之前已经证明,使用 Milstein 的 Ru(PNN) 钳配合物作为催化剂,醇很容易以 1,4-方式添加到这些底物中。然而,尝试将水添加到 α,β-不饱和腈中,得到的 3-羟基腈收率平平。另一方面,苯甲醇的加成反应对各种β-取代不饱和腈的产率都很高。随后用 TMSCl/FeCl 3处理苯甲醇加成产物,形成 3-羟基烷基腈。由 oxa-Michael 加成得到的 3-苄氧基-烷基腈也可以在酸存在下直接氢化,以优异的收率得到 HCl 盐形式的氨基醇。在中性条件下氢化得到仲胺和叔胺的混合物。在碱和 Boc 酸酐存在下氢化,得到正交双保护的氨基醇,其中苄基醚随后可裂解生成 Boc 保护的氨基醇。因此,从苯甲醇与 α,β-不饱和腈的 oxa-Michael 加成开始,可以得到各种O和N官能团之间具有 1,3-关系的分子支架。































 京公网安备 11010802027423号
京公网安备 11010802027423号