当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unsaturated Derivatives of Trifluoromethanesulfonamide
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-04-10 , DOI: 10.1002/ejoc.201800130 Bagrat A. Shainyan 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-04-10 , DOI: 10.1002/ejoc.201800130 Bagrat A. Shainyan 1
Affiliation
|
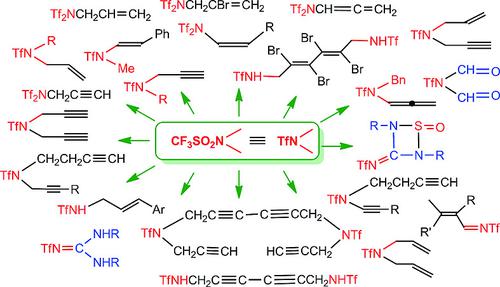
Unsaturated derivatives of trifluoromethanesulfonamide (CF3SO2NH2, triflamide, TfNH2) of general formula TfNRR′ where R and/or R′ contain C=C or C≡C bonds, are of particular interest in triflate chemistry because they open the way for further functionalization and provide access to new adducts and cycloadducts. Two approaches to these compounds have been developed in our group. One is treatment of triflic anhydride (or fluoride) with unsaturated amines, or alkylation of triflamide with unsaturated alkyl halides. The second approach consists of oxidative triflamidation of dienes, with one C=C bond remaining intact. Another class of unsaturated triflamide derivatives is that of N‐alkylidenetriflamides (TfN=R). At the time our comprehensive review on triflamides and related compounds covering the literature up to 2012 was published, very little information on unsaturated triflamides was available. Since then, research in this area has expanded. In this review we demonstrate new examples of unusual reactivity of unsaturated triflamides, such as unprecedented splitting of α,ω‐bis(triflamidomethyl)diacetylene, diverse courses of oxidative triflamidation and arenesulfonamidation of dienes, different routes of cycloaddition of fluorinated and non‐fluorinated N‐sulfinylsulfonamides to carbodiimides, isomerization, etc. The high NH‐acidity, the strong electron‐acceptor ability and good nucleofugality of the triflyl and triflamido groups, and the presence of multiple bonds in the unsaturated triflamides all make them both interesting objects for investigations of hydrogen bonding and self‐organization processes, as well as valuable synthons in organic chemistry.
中文翻译:

三氟甲磺酰胺的不饱和衍生物
通式为TfNRR'的三氟甲磺酰胺的不饱和衍生物(CF 3 SO 2 NH 2,三氟化物,TfNH 2),其中R和/或R'包含C = C或C≡C键,在三氟甲磺酸化学中特别受关注,因为它们打开了进一步功能化的途径,并提供获得新加合物和环加合物的途径。我们小组已开发出两种处理这些化合物的方法。一种是用不饱和胺处理三氟甲磺酸酐(或氟化物),或用不饱和烷基卤化物将三氟化物烷基化。第二种方法包括二烯的氧化三氟化,其中一个C = C键保持完整。另一类不饱和三氟化物衍生物是N‐烷基亚胺基三氟化物(TfN = R)。在我们发表有关2012年文献的有关三氟化物及相关化合物的全面综述之时,关于不饱和三氟化物的信息很少。从那时起,在这一领域的研究就扩大了。在这篇综述中,我们展示了不饱和三氟化物异常反应性的新实例,例如前所未有的α,ω-双(三氟甲基)双乙炔裂解,氧化三氟化和二烯芳烃磺酰胺化的不同过程,氟化和非氟化N的不同环加成途径-亚磺酰基磺酰胺转化为碳二亚胺,异构化等。三氟甲基和三氟烷基的高NH酸度,强电子受体能力和良好的核键性,以及不饱和三氟化合物中存在多个键都使它们成为研究以下两个有趣的对象氢键和自组织过程,以及有机化学中有价值的合成子。
更新日期:2018-07-14
中文翻译:

三氟甲磺酰胺的不饱和衍生物
通式为TfNRR'的三氟甲磺酰胺的不饱和衍生物(CF 3 SO 2 NH 2,三氟化物,TfNH 2),其中R和/或R'包含C = C或C≡C键,在三氟甲磺酸化学中特别受关注,因为它们打开了进一步功能化的途径,并提供获得新加合物和环加合物的途径。我们小组已开发出两种处理这些化合物的方法。一种是用不饱和胺处理三氟甲磺酸酐(或氟化物),或用不饱和烷基卤化物将三氟化物烷基化。第二种方法包括二烯的氧化三氟化,其中一个C = C键保持完整。另一类不饱和三氟化物衍生物是N‐烷基亚胺基三氟化物(TfN = R)。在我们发表有关2012年文献的有关三氟化物及相关化合物的全面综述之时,关于不饱和三氟化物的信息很少。从那时起,在这一领域的研究就扩大了。在这篇综述中,我们展示了不饱和三氟化物异常反应性的新实例,例如前所未有的α,ω-双(三氟甲基)双乙炔裂解,氧化三氟化和二烯芳烃磺酰胺化的不同过程,氟化和非氟化N的不同环加成途径-亚磺酰基磺酰胺转化为碳二亚胺,异构化等。三氟甲基和三氟烷基的高NH酸度,强电子受体能力和良好的核键性,以及不饱和三氟化合物中存在多个键都使它们成为研究以下两个有趣的对象氢键和自组织过程,以及有机化学中有价值的合成子。















































 京公网安备 11010802027423号
京公网安备 11010802027423号