当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Au11(PPh2Py)7Br3 for the Highly Chemoselective Hydrogenation of Nitrobenzaldehyde
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-12-01 00:00:00 , DOI: 10.1021/acscatal.5b02116
Chao Liu 1 , Hadi Abroshan 2 , Chunyang Yan 1 , Gao Li 1 , Masatake Haruta 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-12-01 00:00:00 , DOI: 10.1021/acscatal.5b02116
Chao Liu 1 , Hadi Abroshan 2 , Chunyang Yan 1 , Gao Li 1 , Masatake Haruta 1
Affiliation
|
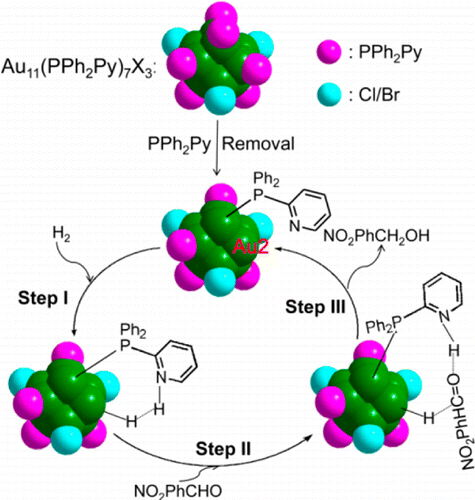
In this study, the gold clusters Au11(PPh3)7Cl3 and Au11(PPh2Py)7Br3 (PPh2Py = diphenyl-2-pyridylphosphine) are synthesized via a one-pot procedure based on the wet chemical reduction method. The Au11(PPh3)7Cl3 cluster is found to be active in the chemoselective hydrogenation of 4-nitrobenzaldehyde in the presence of hydrogen (H2) and a base (e.g., pyridine). Interestingly, the cluster with the functional ligand PPh2Py shows similar activity without losing catalytic efficiency in the absence of the base. The structure of the gold clusters and reaction pathway of the catalytic hydrogenation are investigated at the atomic/molecular level via UV–vis spectroscopy, electrospray ionization (ESI) mass spectrometry, and density functional theory (DFT) calculations. It is found that one ligand (PPh3 or PPh2Py) removal is the first step to expose the core of the gold clusters to reactants, providing an active site for the catalytic reaction. Then, the H–H bond of the H2 molecule becomes activated with the aid of either free amine (base) or ligand PPh2Py which is attached to the gold clusters. This work demonstrates the promise of the functional ligand PPh2Py in the catalytic hydrogenation to reduce the amount of materials (free base: e.g., pyridine) that ultimately enter the waste stream, thereby providing a more environmentally friendly reaction medium.
中文翻译:

一锅合成Au 11(PPh 2 Py)7 Br 3用于硝基苯甲醛的高化学选择性加氢
在这项研究中,金簇Au 11(PPh 3)7 Cl 3和Au 11(PPh 2 Py)7 Br 3(PPh 2 Py =二苯基-2-吡啶基膦)通过湿法一锅法合成化学还原法。发现Au 11(PPh 3)7 Cl 3簇在氢(H 2)和碱(例如吡啶)存在下在4-硝基苯甲醛的化学选择性氢化中具有活性。有趣的是,具有功能性配体PPh 2的簇在没有碱的情况下,Py表现出相似的活性,而不会失去催化效率。通过紫外可见光谱,电喷雾电离(ESI)质谱和密度泛函理论(DFT)计算,在原子/分子水平上研究了金团簇的结构和催化加氢的反应途径。已经发现,去除一个配体(PPh 3或PPh 2 Py)是将金簇的核心暴露于反应物的第一步,从而为催化反应提供了一个活性位点。然后,借助于游离胺(碱)或与金簇连接的配体PPh 2 Py,H 2分子的H–H键被激活。这项工作证明了功能性配体PPh的前景2 Py在催化加氢中以减少最终进入废物流的物质(游离碱:例如吡啶)的量,从而提供一种对环境更友好的反应介质。
更新日期:2015-12-01
中文翻译:

一锅合成Au 11(PPh 2 Py)7 Br 3用于硝基苯甲醛的高化学选择性加氢
在这项研究中,金簇Au 11(PPh 3)7 Cl 3和Au 11(PPh 2 Py)7 Br 3(PPh 2 Py =二苯基-2-吡啶基膦)通过湿法一锅法合成化学还原法。发现Au 11(PPh 3)7 Cl 3簇在氢(H 2)和碱(例如吡啶)存在下在4-硝基苯甲醛的化学选择性氢化中具有活性。有趣的是,具有功能性配体PPh 2的簇在没有碱的情况下,Py表现出相似的活性,而不会失去催化效率。通过紫外可见光谱,电喷雾电离(ESI)质谱和密度泛函理论(DFT)计算,在原子/分子水平上研究了金团簇的结构和催化加氢的反应途径。已经发现,去除一个配体(PPh 3或PPh 2 Py)是将金簇的核心暴露于反应物的第一步,从而为催化反应提供了一个活性位点。然后,借助于游离胺(碱)或与金簇连接的配体PPh 2 Py,H 2分子的H–H键被激活。这项工作证明了功能性配体PPh的前景2 Py在催化加氢中以减少最终进入废物流的物质(游离碱:例如吡啶)的量,从而提供一种对环境更友好的反应介质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号