当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bottom‐up Construction of π‐Extended Arenes by a Palladium‐Catalyzed Annulative Dimerization of o‐Iodobiaryl Compounds
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-05-03 , DOI: 10.1002/anie.201803603 Chendan Zhu 1 , Di Wang 1 , Dingyi Wang 1 , Yue Zhao 1 , Wei-Yin Sun 1 , Zhuangzhi Shi 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-05-03 , DOI: 10.1002/anie.201803603 Chendan Zhu 1 , Di Wang 1 , Dingyi Wang 1 , Yue Zhao 1 , Wei-Yin Sun 1 , Zhuangzhi Shi 1
Affiliation
|
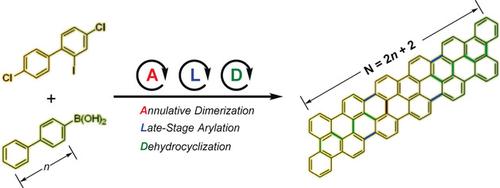
A straightforward method was developed for construction of aromatic compounds with a triphenylene core. The method involves Pd‐catalyzed annulative dimerization of o‐iodobiaryl compounds by double C−I and C−H bond cleavage steps. Simple reaction conditions are needed, requiring neither a ligand nor an oxidant, and the reaction tolerates a wide range of coupling partners without compromising efficiency or scalability. Significantly, the tetrachloro‐substituted synthon, 1,6,11‐trichloro‐4‐(4‐chlorophenyl)triphenylene, can be generated and used to prepare a series of fully fused, small graphene nanoribbons by a late‐stage arylation with arylboronic acids and a subsequent Scholl reaction. The synthetic strategy enables bottom‐up access to extended π‐systems in a controlled manner.
中文翻译:

钯催化的邻碘联芳基化合物的环二聚法从下而上构造π扩展的芳烃
开发了一种简单的方法来构建具有三亚苯基核的芳族化合物。该方法涉及通过碘键和CH键的双键裂解步骤,钯催化的邻碘联芳基化合物的环状二聚化。需要简单的反应条件,既不需要配体也不需要氧化剂,并且该反应在不影响效率或可扩展性的情况下可以耐受多种偶联伙伴。值得注意的是,可以生成四氯取代的合成子1,1,6,11-三氯-4-(4-氯苯基)三亚苯基,并通过与芳基硼酸的后期芳基化反应来制备一系列完全熔融的小石墨烯纳米带。以及随后的Scholl反应。综合策略使自下而上的访问以受控方式访问扩展的π系统。
更新日期:2018-05-03
中文翻译:

钯催化的邻碘联芳基化合物的环二聚法从下而上构造π扩展的芳烃
开发了一种简单的方法来构建具有三亚苯基核的芳族化合物。该方法涉及通过碘键和CH键的双键裂解步骤,钯催化的邻碘联芳基化合物的环状二聚化。需要简单的反应条件,既不需要配体也不需要氧化剂,并且该反应在不影响效率或可扩展性的情况下可以耐受多种偶联伙伴。值得注意的是,可以生成四氯取代的合成子1,1,6,11-三氯-4-(4-氯苯基)三亚苯基,并通过与芳基硼酸的后期芳基化反应来制备一系列完全熔融的小石墨烯纳米带。以及随后的Scholl反应。综合策略使自下而上的访问以受控方式访问扩展的π系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号