当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Electrochemical Properties of Naphthalene Diimide: Implication for Stable and High-Rate Lithium-Ion Battery Electrodes
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.chemmater.8b01304
Yang Shi , Hanmei Tang , Shengli Jiang , Laure V. Kayser , Mingqian Li , Fang Liu 1 , Fei Ji , Darren J. Lipomi , Shyue Ping Ong , Zheng Chen
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.chemmater.8b01304
Yang Shi , Hanmei Tang , Shengli Jiang , Laure V. Kayser , Mingqian Li , Fang Liu 1 , Fei Ji , Darren J. Lipomi , Shyue Ping Ong , Zheng Chen
Affiliation
|
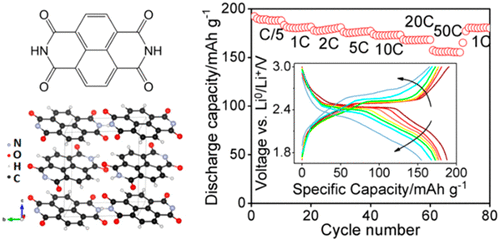
Redox-active organic molecules have attracted much attention as alternatives to transition-metal-based electrodes for lithium-ion batteries due to their low cost and large abundance. However, the relatively low cycling stability still prevents some of the most promising molecules to be used as lithium-ion electrodes. Herein, we used 1,4,5,8-naphthalene diimide (NDI) as a model molecule to systematically investigate its intrinsic electrochemical property, including its electrolyte compatibility, maximum capacity, cycling stability, and rate capability in different organic electrolytes. Extensive physicochemical characterization, electrochemical measurement, and density function theory (DFT) calculation together show that the electrode–electrolyte interaction is the key factor determining its specific capacity and cycling stability. With a proper selection of electrolytes, NDI molecule, which was considered to be not stable for lithium storage, can achieve near theoretical capacity (based on two-electron reaction), very high rate capability, and high cycling stability. This study suggests the importance of understanding the fundamental electrode–electrolyte interactions in designing high-performance organic electrodes.
中文翻译:

了解萘二酰亚胺的电化学性质:对稳定和高速率锂离子电池电极的意义
氧化还原活性有机分子因其低成本和高丰度作为锂离子电池过渡金属基电极的替代品而备受关注。然而,相对较低的循环稳定性仍然阻止了一些最有前途的分子被用作锂离子电极。本文中,我们使用1,4,5,8-萘二酰亚胺(NDI)作为模型分子,系统地研究了其固有的电化学性质,包括其电解质相容性,最大容量,循环稳定性和在不同有机电解质中的速率能力。广泛的理化特性,电化学测量和密度泛函理论(DFT)计算共同表明,电极与电解质的相互作用是决定其比容量和循环稳定性的关键因素。通过正确选择电解质,NDI分子(被认为对于锂存储而言不稳定)可以达到接近理论容量(基于二电子反应),极高的倍率容量和高循环稳定性。这项研究表明,在设计高性能有机电极时,了解基本的电极-电解质相互作用非常重要。
更新日期:2018-04-27
中文翻译:

了解萘二酰亚胺的电化学性质:对稳定和高速率锂离子电池电极的意义
氧化还原活性有机分子因其低成本和高丰度作为锂离子电池过渡金属基电极的替代品而备受关注。然而,相对较低的循环稳定性仍然阻止了一些最有前途的分子被用作锂离子电极。本文中,我们使用1,4,5,8-萘二酰亚胺(NDI)作为模型分子,系统地研究了其固有的电化学性质,包括其电解质相容性,最大容量,循环稳定性和在不同有机电解质中的速率能力。广泛的理化特性,电化学测量和密度泛函理论(DFT)计算共同表明,电极与电解质的相互作用是决定其比容量和循环稳定性的关键因素。通过正确选择电解质,NDI分子(被认为对于锂存储而言不稳定)可以达到接近理论容量(基于二电子反应),极高的倍率容量和高循环稳定性。这项研究表明,在设计高性能有机电极时,了解基本的电极-电解质相互作用非常重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号