当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Studies on the Dirhodium-Catalyzed [3 + 2] and [3 + 3] Cycloaddition Reactions of Enol Diazoacetates with Isoquinolinium Methylide: Mechanism, Selectivity, and Ligand Effect
Organometallics ( IF 2.5 ) Pub Date : 2018-04-27 , DOI: 10.1021/acs.organomet.8b00069 Shi-Jun Li 1 , De-Cai Fang 1
Organometallics ( IF 2.5 ) Pub Date : 2018-04-27 , DOI: 10.1021/acs.organomet.8b00069 Shi-Jun Li 1 , De-Cai Fang 1
Affiliation
|
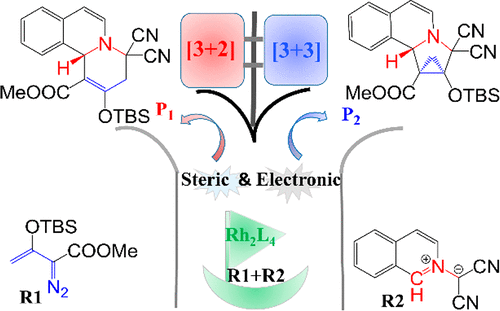
The reaction mechanisms of dirhodium-catalyzed [3 + 2] and [3 + 3] cycloaddition between enol diazoacetate and isoquinolinium methylide have been studied in detail using density functional theory and a solution-phase translational entropy model. The reaction starts with the formation of a metallic carbene intermediate first, from which two competing reaction channels of [3 + 2] and [3 + 3] cycloaddition take place. For CAT1-catalyzed reactions, the calculated activation free energy barriers for [3 + 3] and [3 + 2] cycloaddition reactions are 14.3 and 16.0 kcal mol–1, respectively, which is in good agreement with the ratio of products. Both the steric and electronic effects have been considered for CAT2- and CAT3-catalyzed reactions, with which the ratio of products has also been rationalized.
中文翻译:

DFT研究烯醇重氮乙酸酯与甲基异喹啉鎓甲烷催化的[3 + 2]和[3 + 3]环加成反应:机理,选择性和配体效应
使用密度泛函理论和溶液相平移熵模型,详细研究了烯丙基重氮乙酸酯与甲基异喹啉鎓在二甲基吡啶鎓催化的[3 + 2]和[3 + 3]环加成反应中的反应机理。该反应首先从形成金属卡宾中间体开始,从中发生两个相互竞争的[3 + 2]和[3 + 3]环加成反应通道。对于CAT1催化的反应,计算出的[3 + 3]和[3 + 2]环加成反应的活化自由能垒分别为14.3和16.0 kcal mol –1,这与产物的比例非常吻合。CAT2和CAT3都考虑了空间效应和电子效应-催化的反应,由此也使产物的比例合理化。
更新日期:2018-04-28
中文翻译:

DFT研究烯醇重氮乙酸酯与甲基异喹啉鎓甲烷催化的[3 + 2]和[3 + 3]环加成反应:机理,选择性和配体效应
使用密度泛函理论和溶液相平移熵模型,详细研究了烯丙基重氮乙酸酯与甲基异喹啉鎓在二甲基吡啶鎓催化的[3 + 2]和[3 + 3]环加成反应中的反应机理。该反应首先从形成金属卡宾中间体开始,从中发生两个相互竞争的[3 + 2]和[3 + 3]环加成反应通道。对于CAT1催化的反应,计算出的[3 + 3]和[3 + 2]环加成反应的活化自由能垒分别为14.3和16.0 kcal mol –1,这与产物的比例非常吻合。CAT2和CAT3都考虑了空间效应和电子效应-催化的反应,由此也使产物的比例合理化。































 京公网安备 11010802027423号
京公网安备 11010802027423号