当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Study on the Mechanism of Hydrogen Storage Based on the Formate-Bicarbonate Equilibrium Catalyzed by an Ir-NHC Complex: An Elusive Intramolecular C–H Activation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00382 Péter Pál Fehér 1, 2 , Henrietta Horváth 3 , Ferenc Joó 1, 3 , Mihály Purgel 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00382 Péter Pál Fehér 1, 2 , Henrietta Horváth 3 , Ferenc Joó 1, 3 , Mihály Purgel 1
Affiliation
|
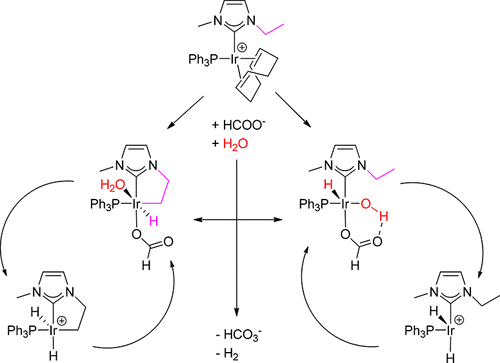
A novel iridium based, water-soluble phosphine-NHC (N-heterocyclic carbene) complex, Na2[Ir(emim)(η4-COD)(mtppts)] was previously developed in our research group. It was shown that it is a very effective catalyst for the reversible storage of hydrogen based on the formate-bicarbonate equilibrium. In this paper, we present a DFT investigation on the noninnocent behavior of the NHC ligand toward C–H activation of the N-ethyl side chain and its possible role in the hydrogen storage mechanism. After preliminary investigations, using both computations and NMR measurements, we conclude that the COD ligand leaves the precatalyst irreversibly and the C–H activation takes place on a monophosphine complex. Two main pathways are considered in which the active Ir(III) complexes are generated differently: One is the cyclometalation path involving the ethyl side chain, the other is the oxidative addition step of a water molecule which has a higher barrier but provide a more stable starting state. We find that though the latter, a catalytic cycle where a hydride is abstracted from formate and gets protonated by solvent molecules gives the lowest calculated energy barrier, +25.8 kcal mol–1. That is, avoiding further redox processes is preferred. There are other pathways involving thermodynamically accessible C–H activated iridacycles but those involve slightly higher overall activation barriers due to the required Ir(I)/Ir(III) transitions. The cycle which involves only iridacycle intermediates offer the lowest energy span (energy difference calculated between only the highest and lowest energy points inside the cycle), however. Together with the experimental results, this implies that C–H activation of the N-ethyl side chain happens off-cycle or the starting solvent addition step of the dominant pathway is blocked kinetically. We also discuss the hydrogen uptake reaction catalyzed by cyclometalated species where the reduction of CO2 is preferred over reversing the first main cycle.
中文翻译:

基于Ir-NHC配合物催化的甲酸酯-碳酸氢盐平衡的储氢机理的DFT研究:分子内CH活化难以实现
一种新的基于铱,水溶性膦- NHC(N-杂环卡宾)络合物,钠2的[Ir(EMIM)(η 4 -COD)(米tppts)]之前是在我们的研究小组中开发的。结果表明,基于甲酸-碳酸氢盐平衡,它是用于氢的可逆存储的非常有效的催化剂。在本文中,我们对DHC研究了NHC配体对N-乙基侧链的C–H活化的无害行为及其在储氢机理中的可能作用。经过初步研究,使用计算和NMR测量,我们得出结论:COD配体不可逆地离开了前催化剂,并且C–H活化发生在单膦配合物上。考虑了两种主要途径,其中活性Ir(III)配合物的生成方式不同:一种是涉及乙基侧链的环金属化途径,另一个是水分子的氧化加成步骤,该步骤具有较高的势垒但提供了更稳定的起始状态。我们发现尽管是后者,但从甲酸盐中提取出氢化物并被溶剂分子质子化的催化循环提供了最低的计算能垒,+ 25.8 kcal mol–1。即,优选避免进一步的氧化还原过程。还有其他途径涉及热力学上可被C–H激活的iridacycles,但由于所需的Ir(I)/ Ir(III)跃迁,这些途径涉及总体活化障碍略高一些。但是,仅涉及iridacycle中间体的循环提供了最低的能量跨度(仅在循环内最高和最低能量点之间计算出的能量差)。连同实验结果一起,这表明N-乙基侧链的C–H活化是非循环发生的,或者主要途径的起始溶剂添加步骤在动力学上受阻。我们还讨论了环金属化物种催化的氢吸收反应,其中CO 2的还原 优先于反转第一个主循环。
更新日期:2018-04-27
中文翻译:

基于Ir-NHC配合物催化的甲酸酯-碳酸氢盐平衡的储氢机理的DFT研究:分子内CH活化难以实现
一种新的基于铱,水溶性膦- NHC(N-杂环卡宾)络合物,钠2的[Ir(EMIM)(η 4 -COD)(米tppts)]之前是在我们的研究小组中开发的。结果表明,基于甲酸-碳酸氢盐平衡,它是用于氢的可逆存储的非常有效的催化剂。在本文中,我们对DHC研究了NHC配体对N-乙基侧链的C–H活化的无害行为及其在储氢机理中的可能作用。经过初步研究,使用计算和NMR测量,我们得出结论:COD配体不可逆地离开了前催化剂,并且C–H活化发生在单膦配合物上。考虑了两种主要途径,其中活性Ir(III)配合物的生成方式不同:一种是涉及乙基侧链的环金属化途径,另一个是水分子的氧化加成步骤,该步骤具有较高的势垒但提供了更稳定的起始状态。我们发现尽管是后者,但从甲酸盐中提取出氢化物并被溶剂分子质子化的催化循环提供了最低的计算能垒,+ 25.8 kcal mol–1。即,优选避免进一步的氧化还原过程。还有其他途径涉及热力学上可被C–H激活的iridacycles,但由于所需的Ir(I)/ Ir(III)跃迁,这些途径涉及总体活化障碍略高一些。但是,仅涉及iridacycle中间体的循环提供了最低的能量跨度(仅在循环内最高和最低能量点之间计算出的能量差)。连同实验结果一起,这表明N-乙基侧链的C–H活化是非循环发生的,或者主要途径的起始溶剂添加步骤在动力学上受阻。我们还讨论了环金属化物种催化的氢吸收反应,其中CO 2的还原 优先于反转第一个主循环。































 京公网安备 11010802027423号
京公网安备 11010802027423号