当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Study of (De)sodiation in alpha-MnO2 nanowires
Nano Energy ( IF 16.8 ) Pub Date : 2015-12-03 06:33:41 Yifei Yuan, Lu Ma, Kun He, Wentao Yao, Anmin Nie, Xuanxuan Bi, Khalil Amine, Tianpin Wu, Jun Lu, Reza Shahbazian-Yassr
Nano Energy ( IF 16.8 ) Pub Date : 2015-12-03 06:33:41 Yifei Yuan, Lu Ma, Kun He, Wentao Yao, Anmin Nie, Xuanxuan Bi, Khalil Amine, Tianpin Wu, Jun Lu, Reza Shahbazian-Yassr
|
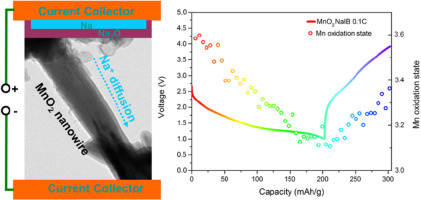
In this report, the electrochemical sodiation and desodiation in single crystalline alpha-MnO2 nanowires are studied dynamically at both single particle level using in situ transmission electron microscopy (TEM) and bulk level using in situ synchrotron X-ray. The TEM results suggest that the first sodiation process starts with tunnel-based Na+ intercalation, experiences the formation of Na0.5MnO2 as a result of tunnel degradation, and ends with the Mn2O3 phase. The inserted Na+ can be partially extracted out of the sodiated products, and the following cycles are dominated by the reversible conversion reaction between Na0.5MnO2 and Mn2O3. The Mn valence evolution inside a cycling coin using alpha-MnO2 nanowire electrode also exhibits partially reversible characteristic, agreeing well with the in situ TEM analysis. The sodiation is compared with lithiation in the same alpha-MnO2 nanowires. Both Na+ and Li+ interact with the tunneled structure via a similar tunnel-driven intercalation mechanism before Mn4+ is reduced to Mn3.5+. For the following deep insertion, the tunnels survive up to LiMnO2 (Mn3+) during lithiation, while the sodiation proceeds via a different mechanism that involves obvious phase transition and fast tunnel degradation after Mn’s valence is below 3.5+. The difference in charge carrier insertion mechanisms can be ascribed to the strong interaction between the tunnel frame and inserted Na+ possessing a larger ionic size than inserted Li+.
中文翻译:

α-MnO2纳米线中(去)钠盐的动态研究
在该报告中,在单结晶的α-MnO的电化学sodiation和desodiation 2纳米线在使用原位透射电子显微镜(TEM)和体用原位同步辐射X射线级单粒子水平动态研究。TEM结果表明,第一个成膜过程始于基于隧道的Na +嵌入,经历了由于隧道降解而形成的Na 0.5 MnO 2,并终止于Mn 2 O 3相。插入的Na +可以部分地从水合产物中提取出来,随后的循环主要由Na 0.5 MnO之间的可逆转化反应决定。2和Mn 2 O 3。使用alpha-MnO 2纳米线电极的循环硬币内部的Mn价演化也表现出部分可逆的特性,与原位TEM分析非常吻合。该sodiation与在相同的α-锂化的MnO相比2纳米线。在Mn 4+还原为Mn 3.5+之前,Na +和Li +都通过类似的隧道驱动插层机制与隧道结构相互作用。对于随后的深度插入,隧道可以存活到LiMnO 2(Mn 3+)在锂化过程中,而固着作用是通过另一种机制进行的,该机制涉及明显的相变和Mn价低于3.5+后快速的隧道降解。电荷载体插入机理的差异可以归结为隧道框架与插入的Na +之间的强相互作用,其离子尺寸大于插入的Li +。
更新日期:2015-12-03
中文翻译:

α-MnO2纳米线中(去)钠盐的动态研究
在该报告中,在单结晶的α-MnO的电化学sodiation和desodiation 2纳米线在使用原位透射电子显微镜(TEM)和体用原位同步辐射X射线级单粒子水平动态研究。TEM结果表明,第一个成膜过程始于基于隧道的Na +嵌入,经历了由于隧道降解而形成的Na 0.5 MnO 2,并终止于Mn 2 O 3相。插入的Na +可以部分地从水合产物中提取出来,随后的循环主要由Na 0.5 MnO之间的可逆转化反应决定。2和Mn 2 O 3。使用alpha-MnO 2纳米线电极的循环硬币内部的Mn价演化也表现出部分可逆的特性,与原位TEM分析非常吻合。该sodiation与在相同的α-锂化的MnO相比2纳米线。在Mn 4+还原为Mn 3.5+之前,Na +和Li +都通过类似的隧道驱动插层机制与隧道结构相互作用。对于随后的深度插入,隧道可以存活到LiMnO 2(Mn 3+)在锂化过程中,而固着作用是通过另一种机制进行的,该机制涉及明显的相变和Mn价低于3.5+后快速的隧道降解。电荷载体插入机理的差异可以归结为隧道框架与插入的Na +之间的强相互作用,其离子尺寸大于插入的Li +。































 京公网安备 11010802027423号
京公网安备 11010802027423号