Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response.
Cell ( IF 45.5 ) Pub Date : 2018-May-03 , DOI: 10.1016/j.cell.2018.03.064 Minghong Jiang , Shikun Zhang , Zongheng Yang , Hongyu Lin , Jun Zhu , Lun Liu , Wendie Wang , Shuo Liu , Wei Liu , Yuanwu Ma , Lianfeng Zhang , Xuetao Cao
Cell ( IF 45.5 ) Pub Date : 2018-May-03 , DOI: 10.1016/j.cell.2018.03.064 Minghong Jiang , Shikun Zhang , Zongheng Yang , Hongyu Lin , Jun Zhu , Lun Liu , Wendie Wang , Shuo Liu , Wei Liu , Yuanwu Ma , Lianfeng Zhang , Xuetao Cao
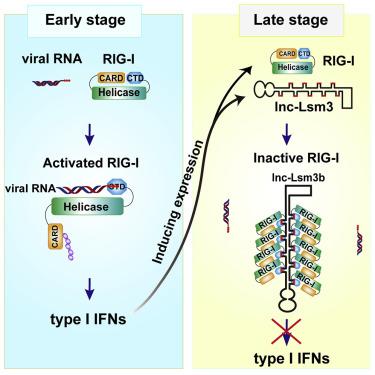
|
The innate RNA sensor RIG-I is critical in the initiation of antiviral type I interferons (IFNs) production upon recognition of "non-self" viral RNAs. Here, we identify a host-derived, IFN-inducible long noncoding RNA, lnc-Lsm3b, that can compete with viral RNAs in the binding of RIG-I monomers and feedback inactivate the RIG-I innate function at late stage of innate response. Mechanistically, binding of lnc-Lsm3b restricts RIG-I protein's conformational shift and prevents downstream signaling, thereby terminating type I IFNs production. Multivalent structural motifs and long-stem structure are critical features of lnc-Lsm3b for RIG-I binding and inhibition. These data reveal a non-canonical self-recognition mode in the regulation of immune response and demonstrate an important role of an inducible "self" lncRNA acting as a potent molecular decoy actively saturating RIG-I binding sites to restrict the duration of "non-self" RNA-induced innate immune response and maintaining immune homeostasis, with potential utility in inflammatory disease management.
中文翻译:

RIG-I反馈对诱导型宿主lncRNA的自我识别可限制先天免疫反应。
固有的RNA传感器RIG-I在识别“非自身”病毒RNA后启动抗病毒I型干扰素(IFN)产生中至关重要。在这里,我们确定了宿主衍生的,IFN诱导的长非编码RNA,lnc-Lsm3b,可以与病毒RNA竞争RIG-I单体的结合,并且在先天性反应的后期反馈使RIG-I的先天功能失活。从机制上讲,lnc-Lsm3b的结合限制了RIG-1蛋白的构象变化并阻止了下游信号传导,从而终止了I型IFN的产生。多价结构基序和长茎结构是lnc-Lsm3b对于RIG-I结合和抑制的关键特征。这些数据揭示了在调节免疫反应中的非典型自我识别模式,并证明了诱导型“自我”的重要作用。
更新日期:2018-04-27
中文翻译:

RIG-I反馈对诱导型宿主lncRNA的自我识别可限制先天免疫反应。
固有的RNA传感器RIG-I在识别“非自身”病毒RNA后启动抗病毒I型干扰素(IFN)产生中至关重要。在这里,我们确定了宿主衍生的,IFN诱导的长非编码RNA,lnc-Lsm3b,可以与病毒RNA竞争RIG-I单体的结合,并且在先天性反应的后期反馈使RIG-I的先天功能失活。从机制上讲,lnc-Lsm3b的结合限制了RIG-1蛋白的构象变化并阻止了下游信号传导,从而终止了I型IFN的产生。多价结构基序和长茎结构是lnc-Lsm3b对于RIG-I结合和抑制的关键特征。这些数据揭示了在调节免疫反应中的非典型自我识别模式,并证明了诱导型“自我”的重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号